- Record: found
- Abstract: found
- Article: found
Size-Dependent Cytotoxicity, Adhesion, and Endocytosis of Micro-/Nano-hydroxyapatite Crystals in HK-2 Cells
Read this article at
Abstract

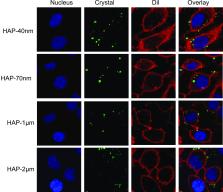
Nano-hydroxyapatite (nano-HAP) is often used as a crystal nest to induce calcium oxalate (CaOx) kidney stone formation, but the mechanism of interaction between HAP crystals of different properties and renal tubular epithelial cells remains unclear. In this study, the adhesion and endocytosis of HAP crystals with sizes of 40 nm, 70 nm, 1 μm, and 2 μm (HAP-40 nm, HAP-70 nm, HAP-1 μm, and HAP-2 μm, respectively) to human renal proximal tubular epithelial cells (HK-2) were comparatively studied. The results showed that HAP crystals of all sizes promoted the expression of osteopontin and hyaluronic acid on the cell surface, destroyed the integrity of the lysosomes, and induced the apoptosis and necrosis of cells. Nano-HAP crystals had a higher specific surface area, a smaller contact angle, a higher surface energy, and a lower Zeta potential than those of micro-HAP. Therefore, the abilities of HK-2 cells to adhere to and endocytose nano-HAP crystals were greater than their abilities to do the same for micro-HAP crystals. The order of the endocytosed crystals was as follows: HAP-40 nm > HAP-70 nm > HAP-1 μm > HAP-2 μm. The endocytosed HAP crystals entered the lysosomes. The more crystal endocytosis and adhesion there is, the more toxic it is to HK-2 cells. The results of this study showed that nanosized HAP crystals greatly promoted the formation of kidney stones than micrometer-sized HAP crystals.
Related collections
Most cited references43
- Record: found
- Abstract: found
- Article: not found
Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells.
- Record: found
- Abstract: found
- Article: not found
The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function.
- Record: found
- Abstract: not found
- Article: not found