- Record: found
- Abstract: found
- Article: found
ICG-Fluorescence Imaging for Margin Assessment During Minimally Invasive Colorectal Liver Metastasis Resection
Read this article at
Key Points
Question
Is near-infrared fluorescence imaging with indocyanine green (ICG) associated with improved oncologic resections in patients undergoing minimally invasive resections of colorectal liver metastases?
Abstract
Importance
Unintended tumor-positive resection margins occur frequently during minimally invasive surgery for colorectal liver metastases and potentially negatively influence oncologic outcomes.
Objective
To assess whether indocyanine green (ICG)–fluorescence–guided surgery is associated with achieving a higher radical resection rate in minimally invasive colorectal liver metastasis surgery and to assess the accuracy of ICG fluorescence for predicting the resection margin status.
Design, Setting, and Participants
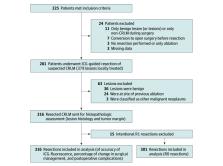
The MIMIC (Minimally Invasive, Indocyanine-Guided Metastasectomy in Patients With Colorectal Liver Metastases) trial was designed as a prospective single-arm multicenter cohort study in 8 Dutch liver surgery centers. Patients were scheduled to undergo minimally invasive (laparoscopic or robot-assisted) resections of colorectal liver metastases between September 1, 2018, and June 30, 2021.
Exposures
All patients received a single intravenous bolus of 10 mg of ICG 24 hours prior to surgery. During surgery, ICG-fluorescence imaging was used as an adjunct to ultrasonography and regular laparoscopy to guide and assess the resection margin in real time. The ICG-fluorescence imaging was performed during and after liver parenchymal transection to enable real-time assessment of the tumor margin. Absence of ICG fluorescence was favorable both during transection and in the tumor bed directly after resection.
Main Outcomes and Measures
The primary outcome measure was the radical (R0) resection rate, defined by the percentage of colorectal liver metastases resected with at least a 1 mm distance between the tumor and resection plane. Secondary outcomes were the accuracy of ICG fluorescence in detecting margin-positive (R1; <1 mm margin) resections and the change in surgical management.
Results
In total, 225 patients were enrolled, of whom 201 (116 [57.7%] male; median age, 65 [IQR, 57-72] years) with 316 histologically proven colorectal liver metastases were included in the final analysis. The overall R0 resection rate was 92.4%. Re-resection of ICG-fluorescent tissue in the resection cavity was associated with a 5.0% increase in the R0 percentage (from 87.4% to 92.4%; P < .001). The sensitivity and specificity for real-time resection margin assessment were 60% and 90%, respectively (area under the receiver operating characteristic curve, 0.751; 95% CI, 0.668-0.833), with a positive predictive value of 54% and a negative predictive value of 92%. After training and proctoring of the first procedures, participating centers that were new to the technique had a comparable false-positive rate for predicting R1 resections during the first 10 procedures (odds ratio, 1.36; 95% CI, 0.44-4.24). The ICG-fluorescence imaging was associated with changes in intraoperative surgical management in 56 (27.9%) of the patients.
Conclusions and Relevance
In this multicenter prospective cohort study, ICG-fluorescence imaging was associated with an increased rate of tumor margin–negative resection and changes in surgical management in more than one-quarter of the patients. The absence of ICG fluorescence during liver parenchymal transection predicted an R0 resection with 92% accuracy. These results suggest that use of ICG fluorescence may provide real-time feedback of the tumor margin and a higher rate of complete oncologic resection.
Abstract
This cohort study of patients undergoing minimally invasive colorectal liver metastasis resections assesses the use of indocyanine green–fluorescence imaging to achieve a higher radical resection rate and greater accuracy in estimating the resection tumor-margin status.
Related collections
Most cited references40
- Record: found
- Abstract: not found
- Article: not found
World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects.
- Record: found
- Abstract: found
- Article: not found
Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey.
- Record: found
- Abstract: found
- Article: not found