- Record: found
- Abstract: found
- Article: found
A group-based mental health intervention for young people living with HIV in Tanzania: results of a pilot individually randomized group treatment trial
Read this article at
Abstract
Background
Increasing numbers of young people living with HIV (YPLWH) have unaddressed mental health challenges. Such challenges are associated with poor antiretroviral therapy (ART) adherence and high mortality. Few evidence-based mental health interventions exist to improve HIV outcomes among YPLWH.
Methods
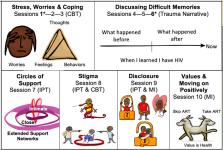
This pilot group treatment trial individually randomized YPLWH from two clinical sites in Tanzania, evaluated acceptability, feasibility, and preliminary effectiveness of a mental health intervention, Sauti ya Vijana (SYV; The Voice of Youth ), was compared to the local standard-of-care (SOC) for improving ART adherence and virologic suppression. Enrolled YPLWH were 12–24 years of age and responded to mental health and stigma questionnaires, self-reported adherence, objective adherence measures (ART concentration in hair), and HIV RNA at baseline and 6-months (post-intervention). Feasibility and acceptability were evaluated, and potential effectiveness was assessed by comparing outcomes between arms using mixed effects modeling.
Results
Between June 2016 and July 2017, 128 YPLWH enrolled; 105 were randomized and 93 (55 in SYV) followed-up at 6-months and were thereby included in this analysis . Mean age was 18.1 years; 51% were female; and 84% were HIV-infected perinatally. Attendance to intervention sessions was 86%; 6-month follow-up was 88%, and fidelity to the protocol approached 100%. Exploratory analyses of effectiveness demonstrated self-reported adherence improved by 7.3 percentage points (95% CI: 2.2, 12.3); and the pooled standard deviation for all ART concentration values increased by 0.17 units (95% CI: − 0.52, 0.85) in the SYV arm compared to SOC. Virologic suppression rates (HIV RNA < 400 copies/mL) at baseline were 65% in both arms but increased to 75% in the SYV arm while staying the same in the SOC arm (RR 1.13; 95% CI: 0.94, 1.36).
Conclusions
YPLWH often have poor HIV outcomes, making interventions to improve outcomes in this population critical. This pilot trial of the Tanzania-based SYV intervention demonstrated trends towards improvement in ART adherence and virologic outcomes among YPLWH, supporting efforts to scale the intervention into a fully-powered effectiveness trial.
Trial registration
ClinicalTrials.gov Identifier: NCT02888288. Registered August 9, 2016. Retrospectively registered as first participant enrolled June 16, 2016.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Validity of the Patient Health Questionnaire-9 for depression screening and diagnosis in East Africa.
- Record: found
- Abstract: found
- Article: not found
Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa.
- Record: found
- Abstract: found
- Article: not found