- Record: found
- Abstract: found
- Article: found
Glia Crosstalk in Neuroinflammatory Diseases
Read this article at
Abstract
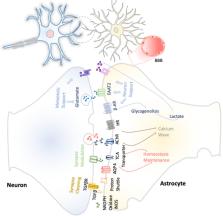
Neuroinflammation constitutes a fundamental cellular process to signal the loss of brain homeostasis. Glial cells play a central role in orchestrating these neuroinflammation processes in both deleterious and beneficial ways. These cellular responses depend on their intercellular interactions with neurons, astrocytes, the blood–brain barrier (BBB), and infiltrated T cells in the central nervous system (CNS). However, this intercellular crosstalk seems to be activated by specific stimuli for each different neurological scenario. This review summarizes key studies linking neuroinflammation with certain neurodegenerative diseases such as Alzheimer disease (AD), Parkinson disease (PD), and amyotrophic lateral sclerosis (ALS) and for the development of better therapeutic strategies based on immunomodulation.
Related collections
Most cited references88
- Record: found
- Abstract: found
- Article: not found
Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha.
- Record: found
- Abstract: found
- Article: not found
Molecular biology of amyotrophic lateral sclerosis: insights from genetics.
- Record: found
- Abstract: found
- Article: not found