- Record: found
- Abstract: found
- Article: found
Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review
Read this article at
Abstract
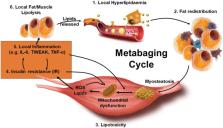
Age‐associated obesity and muscle atrophy (sarcopenia) are intimately connected and are reciprocally regulated by adipose tissue and skeletal muscle dysfunction. During ageing, adipose inflammation leads to the redistribution of fat to the intra‐abdominal area (visceral fat) and fatty infiltrations in skeletal muscles, resulting in decreased overall strength and functionality. Lipids and their derivatives accumulate both within and between muscle cells, inducing mitochondrial dysfunction, disturbing β‐oxidation of fatty acids, and enhancing reactive oxygen species (ROS) production, leading to lipotoxicity and insulin resistance, as well as enhanced secretion of some pro‐inflammatory cytokines. In turn, these muscle‐secreted cytokines may exacerbate adipose tissue atrophy, support chronic low‐grade inflammation, and establish a vicious cycle of local hyperlipidaemia, insulin resistance, and inflammation that spreads systemically, thus promoting the development of sarcopenic obesity (SO). We call this the metabaging cycle. Patients with SO show an increased risk of systemic insulin resistance, systemic inflammation, associated chronic diseases, and the subsequent progression to full‐blown sarcopenia and even cachexia. Meanwhile in many cardiometabolic diseases, the ostensibly protective effect of obesity in extremely elderly subjects, also known as the ‘obesity paradox’, could possibly be explained by our theory that many elderly subjects with normal body mass index might actually harbour SO to various degrees, before it progresses to full‐blown severe sarcopenia. Our review outlines current knowledge concerning the possible chain of causation between sarcopenia and obesity, proposes a solution to the obesity paradox, and the role of fat mass in ageing.
Related collections
Most cited references118
- Record: found
- Abstract: found
- Article: not found
Sarcopenia: European consensus on definition and diagnosis
- Record: found
- Abstract: found
- Article: not found
The NALP3/NLRP3 Inflammasome Instigates Obesity-Induced Autoinflammation and Insulin Resistance
- Record: found
- Abstract: found
- Article: not found