- Record: found
- Abstract: found
- Article: found
Distinct roles of ADIPOR1 and ADIPOR2: A pan-cancer analysis

Read this article at
Abstract
Introduction
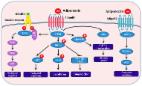
AdipoR1 and AdipoR2 proteins, encoded by ADIPOR1 and ADIPOR2 genes respectively, are the receptors of adiponectin secrected by adipose tissue. Increasing studies have identified the vital role of adipose tissue in various diseases, including cancers. Hence, there is an urgent need to explore the roles of AdipoR1 and AdipoR2 in cancers.
Methods
We conducted a comprehensive pan-cancer analysis for the roles of AdipoR1 and AdipoR2 via several public databases, including expression differences, prognostic value, and the correlations with tumor microenvironment, epigenetic modification, and drug sensitivity.
Results
Both ADIPOR1 and ADIPOR2 genes are dysregulated in most cancers, but their genomic alteration frequencies are low. In addition, they are also correlated with the prognosis of some cancers. Although they are not strongly correlated with tumor mutation burden (TMB) or microsatellite instability (MSI), ADIPOR1/2 genes display a significant association with cancer stemness, tumor immune microenvironment, immune checkpoint genes (especially CD274 and NRP1), and drug sensitivity.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Human Primary Liver Cancer -derived Organoid Cultures for disease modelling and drug screening

- Record: found
- Abstract: found
- Article: found
CancerSEA: a cancer single-cell state atlas
- Record: found
- Abstract: found
- Article: found