- Record: found
- Abstract: found
- Article: found
Association between shared medical appointments and weight loss outcomes and anti‐obesity medication use in patients with obesity
Read this article at
Summary
Objective
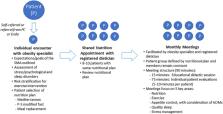
In shared medical appointments (SMAs), multiple patients with a similar clinical diagnosis are seen by a multidisciplinary team for interactive group sessions. Very few studies have specifically studied SMAs and weight loss in patients with obesity. This study compared weight loss outcomes and anti‐obesity medication (AOM) access between patients with obesity managed through (SMAs) versus individual appointments.
Methods
Retrospective study of adults seen for obesity between September 2014 and February 2017 at Cleveland Clinic Institute of Endocrinology and Metabolism. Percent weight loss from baseline was compared between two propensity score‐matched populations: patients who attended ≥1 SMA and patients managed with individual medical appointments.
Results
From all eligible patients identified (n=310 SMA, n=1,993 non‐SMA), 301 matched pairs were evaluated for weight loss. The SMA group (n=301) lost a mean of 4.2%, 5.2% and 3.8% of baseline weight over 6, 12 and 24 months; the non‐SMA group (n=301) lost significantly less weight (1.5%, 1.8% and 1.6%, respectively) (paired t‐test, P<.05). All patients were eligible for US Food and Drug Administration‐approved AOMs based on obesity diagnosis; however, 49.8% (150/301) of matched SMA patients were prescribed an AOM versus 12.3% (37/301) of matched non‐SMA patients.
Conclusion
This study suggests that SMAs may offer a promising alterative for obesity management and one that may facilitate greater utilization of AOMs. In propensity score‐matched cohorts, SMAs were associated with greater weight loss outcomes when compared to usual care facilitated through individual medical appointments alone.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial.
- Record: found
- Abstract: found
- Article: not found
Long-term drug treatment for obesity: a systematic and clinical review.
- Record: found
- Abstract: found
- Article: not found