- Record: found
- Abstract: found
- Article: found
The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines
Read this article at
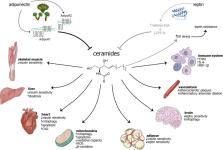
Abstract
Metabolic dysfunction is intertwined with the pathophysiology of both diabetes and cardiovascular disease. Recently, one particular lipid class has been shown to influence the development and sustainment of these diseases: ceramides. As a subtype of sphingolipids, these species are particularly central to many sphingolipid pathways. Increased levels of ceramides are known to correlate with impaired cardiovascular and metabolic health. Furthermore, the interaction between ceramides and adipokines, most notably adiponectin and leptin, appears to play a role in the pathophysiology of these conditions. Adiponectin appears to counteract the detrimental effects of elevated ceramides, largely through activation of the ceramidase activity of its receptors. Elevated ceramides appear to worsen leptin resistance, which is an important phenomenon in the pathophysiology of obesity and metabolic syndrome.
Related collections
Most cited references183
- Record: found
- Abstract: found
- Article: not found
Positional cloning of the mouse obese gene and its human homologue.
- Record: found
- Abstract: found
- Article: not found
Cloning of adiponectin receptors that mediate antidiabetic metabolic effects.
- Record: found
- Abstract: found
- Article: not found