- Record: found
- Abstract: found
- Article: found
Hypertensive Disorders in Pregnancy and Mortality at Delivery Hospitalization — United States, 2017–2019
research-article
Nicole D. Ford , PhD
1
,
,
Shanna Cox , MSPH
1 ,
Jean Y. Ko , PhD
1 ,
Lijing Ouyang , PhD
1 ,
Lisa Romero , DrPh
1 ,
Tiffany Colarusso , MD
1 ,
Cynthia D. Ferre , MA
1 ,
Charlan D. Kroelinger , PhD
1 ,
Donald K. Hayes , MD
2 ,
Wanda D. Barfield , MD
1
29 April 2022
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
Hypertensive disorders in pregnancy (HDPs), defined as prepregnancy (chronic) or pregnancy-associated
hypertension, are common pregnancy complications in the United States.* HDPs are strongly
associated with severe maternal complications, such as heart attack and stroke (
1
), and are a leading cause of pregnancy-related death in the United States.
†
CDC analyzed nationally representative data from the National Inpatient Sample to
calculate the annual prevalence of HDP among delivery hospitalizations and by maternal
characteristics, and the percentage of in-hospital deaths with an HDP diagnosis code
documented. During 2017–2019, the prevalence of HDP among delivery hospitalizations
increased from 13.3% to 15.9%. The prevalence of pregnancy-associated hypertension
increased from 10.8% in 2017 to 13.0% in 2019, while the prevalence of chronic hypertension
increased from 2.0% to 2.3%. Prevalence of HDP was highest among delivery hospitalizations
of non-Hispanic Black or African American (Black) women, non-Hispanic American Indian
and Alaska Native (AI/AN) women, and women aged ≥35 years, residing in zip codes in
the lowest median household income quartile, or delivering in hospitals in the South
or the Midwest Census regions. Among deaths that occurred during delivery hospitalization,
31.6% had any HDP documented. Clinical guidance for reducing complications from HDP
focuses on prompt identification and preventing progression to severe maternal complications
through timely treatment (
1
). Recommendations for identifying and monitoring pregnant persons with hypertension
include measuring blood pressure throughout pregnancy,
§
including self-monitoring. Severe complications and mortality from HDP are preventable
with equitable implementation of strategies to identify and monitor persons with HDP
(
1
) and quality improvement initiatives to improve prompt treatment and increase awareness
of urgent maternal warning signs (
2
).
Delivery hospitalization data for 2017–2019 were analyzed from the National Inpatient
Sample, a nationally representative sample of all U.S. hospital discharges.
¶
CDC identified delivery hospitalizations among females aged 12–55 years using International
Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis
and procedure codes pertaining to delivery and diagnosis-related group delivery codes.**
HDPs were categorized using ICD-10-CM diagnosis codes
††
for chronic hypertension,
§§
pregnancy-associated hypertension,
¶¶
and unspecified maternal hypertension. Deaths were identified based on patient hospital
discharge disposition.
Weighted annual prevalence (percentage) and 95% CI for HDP overall and by each type
were calculated. Change in annual prevalence of HDP overall and by type was assessed
using a linear trend test. Pooling data from this period, CDC calculated the weighted
prevalence and 95% CIs for HDP by selected maternal characteristics (i.e., age group,
race and ethnicity, and primary payer at delivery hospitalization) and characteristics
of the community in which they lived (i.e., county-level rural-urban classification,
zip code–level median household income, and hospital region).*** Rao-Scott chi-square
tests of independence were used to assess whether HDP prevalence differed by characteristics.
Percentage of deaths during delivery hospitalization with a documented HDP diagnosis
code were calculated. All analyses were conducted using SAS software (version 9.4;
SAS Institute); SAS survey procedures and weighting were used to account for complex
sampling in the National Inpatient Sample. This activity was reviewed by CDC and was
conducted consistent with applicable federal law and CDC policy.
†††
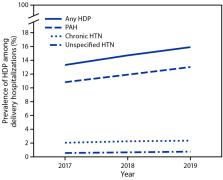
During 2017–2019, the prevalence of HDP among delivery hospitalizations increased
from 13.3% to 15.9% (Figure 1), an increase of approximately 1 percentage point annually.
Linear trend tests suggested that change in annual prevalence of HDP overall, pregnancy-associated
hypertension, and chronic hypertension increased during 2017–2019, while prevalence
of unspecified maternal hypertension remained stable. The prevalence of pregnancy-associated
hypertension increased from 10.8% to 13.0% and that of chronic hypertension increased
from 2.0% to 2.3%.
FIGURE 1
Prevalence of hypertensive disorders in pregnancy* among delivery hospitalizations,
by year — National Inpatient Sample, United States, 2017–2019
Abbreviations: HDP = hypertensive disorder in pregnancy; HTN = hypertension; PAH =
pregnancy-associated hypertension.
* HDPs are defined as chronic hypertension, pregnancy-associated hypertension (i.e.,
gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with superimposed
preeclampsia), and unspecified maternal hypertension.
This figure is a line chart showing the prevalence of hypertensive disorders in pregnancy
among delivery hospitalizations, by year, in the United States during 2017–2019 according
to the National Inpatient Sample.
During 2017–2019 combined, prevalence of HDP overall was 14.6%. Prevalence varied
overall and by HDP type for all maternal characteristics evaluated in the study (Table).
Prevalence of any HDP was higher among delivery hospitalizations to women aged 35–44
(18.0%) and 45–55 years (31.0%) than to younger women, to Black (20.9%) and AI/AN
(16.4%) women than to women of other racial and ethnic groups, to those residing in
rural counties (15.5%) and in zip codes in the lowest median household-level income
quartile (16.4%) than those residing in metropolitan or micropolitan counties or in
zip codes in higher household-level income quartiles, or delivering in hospitals in
the South (15.9%) or Midwest (15.0%) U.S. Census regions than in other Census regions.
These differences in HDP prevalence were similar across HDP types.
TABLE
Prevalence of hypertensive disorders in pregnancy, by patient-, hospital- and zip
code–level characteristics — National Inpatient Sample, United States, 2017–2019
Characteristic
Any hypertensive disorder in pregnancy*
Chronic hypertension
Pregnancy-associated hypertension
Unspecified maternal hypertension
No.†
Row % (95% CI)
No.
Row % (95% CI)
No.
Row % (95% CI)
No.
Row % (95% CI)
Total no. of cases
319,913
—
47,218
—
259,458
—
13,237
—
Maternal age group, yrs
12–24
73,421
13.9 (13.7–14.1)
5,593
1.1 (1.0–1.1)
65,378
12.4 (12.2–12.5)
2,450
0.5 (0.4–0.5)
25–29
85,358
13.5 (13.3–13.7)
10,984
1.7 (1.7–1.8)
71,010
11.2 (11.1–11.4)
3,364
0.5 (0.5–0.6)
30–34
89,242
14.3 (14.1–14.4)
14,982
2.4 (2.3–2.4)
70,287
11.2 (11.1–11.4)
3,973
0.6 (0.6–0.7)
35–44
70,395
18.0 (17.7–18.2)
15,341
3.9 (3.8–4.0)
51,672
13.2 (13.0–13.4)
3,382
0.9 (0.8–0.9)
45–55
1,497
31.0 (29.7–32.4)
318
6.6 (5.9–7.3)
1,111
23.0 (21.8–24.2)
68
1.4 (1.1–1.7)
Race and ethnicity
§
Asian or Pacific Islander
12,183
9.3 (8.8–9.7)
1,616
1.2 (1.1–1.3)
10,134
7.7 (7.3–8.1)
433
0.3 (0.3–0.4)
Black
66,316
20.9 (20.5–21.2)
13,639
4.3 (4.2–4.4)
49,568
15.6 (15.3–15.9)
3,109
1.0 (0.9–1.0)
Hispanic
54,702
12.5 (12.2–12.8)
6,561
1.5 (1.5–1.5)
46,148
10.6 (10.3–10.8)
1,993
0.5 (0.4–0.5)
American Indian and Alaska Native
2,525
16.4 (15.4–17.5)
318
2.1 (1.8–2.3)
2,103
13.7 (12.7–14.6)
104
0.7 (0.5–0.8)
Another race
11,659
12.0 (11.6–12.3)
1,400
1.4 (1.4–1.5)
9,781
10.1 (9.7–10.4)
478
0.5 (0.4–0.5)
White
162,122
14.7 (14.5–14.9)
22,358
2.0 (2.0–2.1)
133,052
12.1 (11.9–12.2)
6,712
0.6 (0.6–0.6)
Missing
10,406
12.7 (12.2–13.1)
1,326
1.6 (1.5–1.7)
8,672
10.6 (10.2–11.0)
408
0.5 (0.4–0.6)
Payer
Public¶
139,227
14.8 (14.6–15.0)
21,541
2.3 (2.2–2.3)
111,543
11.8 (11.7–12.0)
6,143
0.7 (0.6–0.7)
Private insurance
166,455
14.8 (14.7–15.0)
23,826
2.1 (2.1–2.2)
136,153
12.1 (12.0–12.3)
6,476
0.6 (0.6–0.6)
Self-pay/Other
13,837
11.9 (11.6–12.2)
1,791
1.5 (1.5–1.6)
11,443
9.8 (9.5–10.1)
603
0.5 (0.5–0.6)
Rurality (county-level)
Metropolitan
275,342
14.6 (14.4–14.8)
40,136
2.1 (2.1–2.2)
224,232
11.9 (11.7–12.0)
10,974
0.6 (0.6–0.6)
Micropolitan
25,844
14.8 (14.5–15.0)
4,026
2.3 (2.2–2.4)
20,497
11.7 (11.5–11.9)
1,321
0.8 (0.7–0.8)
Rural**
18,139
15.5 (15.1–15.8)
2,980
2.5 (2.4–2.7)
14,241
12.1 (11.9–12.4)
918
0.8 (0.7–0.8)
Median household-level income national quartile for patient zip code
††
Q1
98,661
16.4 (16.1–16.6)
16,218
2.7 (2.6–2.8)
78,022
12.9 (12.7–13.2)
4,421
0.7 (0.7–0.8)
Q2
81,089
14.7 (14.5–14.9)
11,916
2.2 (2.1–2.2)
65,747
11.9 (11.8–12.1)
3,426
0.6 (0.6–0.6)
Q3
77,387
14.4 (14.3–14.6)
10,829
2.0 (2.0–2.1)
63,629
11.9 (11.7–12.0)
2,929
0.5 (0.5–0.6)
Q4
60,014
12.7 (12.5–12.9)
7,830
1.7 (1.6–1.7)
49,857
10.5 (10.3–10.7)
2,327
0.5 (0.5–0.5)
Hospital region
§§
Northeast
48,527
13.9 (13.5–14.4)
6,746
1.9 (1.8–2.0)
40,017
11.5 (11.1–11.9)
1,764
0.5 (0.5–0.5)
Midwest
69,181
15.0 (14.7–15.3)
9,736
2.1 (2.0–2.2)
56,611
12.3 (12.0–12.5)
2,834
0.6 (0.6–0.6)
South
136,435
15.9 (15.7–16.2)
22,355
2.6 (2.5–2.7)
107,940
12.6 (12.4–12.8)
6,140
0.7 (0.7–0.7)
West
65,770
12.7 (12.4–13.0)
8,381
1.6 (1.6–1.7)
54,890
10.6 (10.4–10.9)
2,499
0.5 (0.5–0.5)
Abbreviation: Q = quartile.
* Any hypertensive disorder in pregnancy includes chronic hypertension, pregnancy-associated
hypertension, and unspecified maternal hypertension.
† Numbers are unweighted.
§ Patients with Hispanic ethnicity are classified as Hispanic and all non-Hispanic
patients are classified according to their reported race. The Healthcare Cost and
Utilization Project (HCUP) race and ethnicity category Native American is expressed
as American Indian and Alaska Native.
¶ Public insurance includes Medicare and Medicaid.
** Rural defined as nonmetropolitan and nonmicropolitan counties.
†† 2017 (Q1 = $1–$43,999, Q2 = $44,000–$55,999, Q3 = $56,000–73,999, Q4 = ≥$74,000);
2018 (Q1 = $1–$45,999, Q2 = $46,000–$58,999, Q3 = $59,000–$78,999, Q4 = ≥$79,000);
2019: Q1 = $1–$47,999, Q2 = $48,000–$60,999, Q3 = $61,000–$81,999, Q4 = ≥$82,000.
§§ Hospital region is the census region as defined by the U.S. Census Bureau.
Among maternal deaths that occurred during delivery hospitalization, 31.6% had any
HDP documented and 24.3% had pregnancy-associated hypertension documented. Chronic
or unspecified maternal hypertension was documented in 7.4% of deaths
§§§
(Figure 2).
FIGURE 2
Proportion of deaths* occurring during delivery hospitalization with a documented
diagnosis code of a hypertensive disorder in pregnancy
†
— National Inpatient Sample, United States, 2017–2019
Abbreviation: HDP = hypertensive disorder in pregnancy.
* This study did not assign cause of death but instead examined the proportion of
in-hospital deaths with an HDP diagnosis code documented among delivery hospitalizations.
†
HDPs are defined as chronic hypertension, pregnancy-associated hypertension (i.e.,
gestational hypertension, preeclampsia, eclampsia, and chronic hypertension with superimposed
preeclampsia), and unspecified maternal hypertension. Proportions for chronic and
unspecified maternal hypertension are combined to conform to the Agency for Healthcare
Research and Quality’s data use agreement, which prohibits reporting estimates based
on fewer than 11 unweighted observations.
This figure is a bar chart showing the proportion of deaths occurring during delivery
hospitalization with a documented diagnosis code of a hypertensive disorder in pregnancy
in the United States during 2017–2019— according to the National Inpatient Sample.
Discussion
During 2017–2019, HDPs affected approximately one in seven delivery hospitalizations;
prevalence increases were largely driven by increases in pregnancy-associated hypertension.
HDPs were documented in approximately one in five delivery hospitalizations among
Black women and one in three among women aged 45–55 years. An HDP diagnosis code was
documented in approximately one in three deaths occurring during delivery hospitalization.
Timely diagnosis and treatment of HDP are critical to preventing severe complications
and mortality (
1
).
Prevalence of risk factors for HDP, such as advanced maternal age, obesity, and diabetes
mellitus, have increased in the United States (
1
), and might explain the increase in HDP prevalence. Women with a history of pregnancy-associated
hypertension are at increased risk for cardiovascular disease compared with women
with normotensive pregnancies.
¶¶¶
Addressing risk factors for HDP across the lifespan is important for preventing HDP
and improving future health.****
There are substantial racial and ethnic disparities in HDP prevalence. Compared with
non-Hispanic White women, non-Hispanic Black women have higher odds of entering pregnancy
with chronic hypertension and developing severe preeclampsia (
3
). Factors that contribute to racial and ethnic inequities in chronic and pregnancy-induced
hypertension include higher prevalences of HDP risk factors (
4
), as well as differences in access to health care and the quality of health care
delivered (
5
). Racial bias within the U.S. health care system can affect HDP care from screening
and diagnosis to treatment (
6
). Furthermore, psychosocial stress from experiencing racism is associated with chronic
hypertension (
7
). In a study of racial and ethnic disparities in pregnancy-related deaths, those
caused by HDP among Black and AI/AN women were found to be substantially higher than
those among White women (
8
), highlighting the importance of addressing HDP to reduce inequities in pregnancy-related
mortality.
Regional and rural-urban differences in HDP prevalence have been previously reported
(
9
). Place-based disparities in HDP prevalence might be due to differences in prevalence
of HDP risk factors, including diet, tobacco use, physical activity patterns, poverty,
or access to care.
††††
Rural counties are at higher risk for pregnancy-related mortality than metropolitan
counties (
10
). A strategy to address place-based disparities in HDP and pregnancy-related mortality
can include strengthening regional networks of health care facilities providing risk-appropriate
maternal care through telemedicine and transferring delivery care of persons with
high-risk conditions to facilities that can provide specialty services.
§§§§
Clinical guidance for reducing complications from HDP focuses on prompt identification
and preventing progression to severe maternal complications. Recommendations for identifying
and monitoring pregnant persons with hypertension include measuring blood pressure
throughout pregnancy, including self-monitoring.
¶¶¶¶
Recommendations for preventing preeclampsia include low-dose aspirin for persons at
risk and exercise programs.***** Once a diagnosis of an HDP is received, management
strategies include blood pressure–lowering medication,
†††††
prevention of eclamptic seizures (e.g., administration of magnesium sulfate), and
close maternal and fetal monitoring and coordination and continuity of care during
the postpartum period.
§§§§§
At the systems level, perinatal quality collaboratives (PQCs)
¶¶¶¶¶
implement evidence-based quality improvement initiatives in health care facilities,
including those to address severe hypertension.****** PQCs use collaborative learning,
training, toolkits, and maternal safety bundles (e.g., Alliance for Innovation on
Maternal Health Patient Safety Bundles
††††††
) to improve the quality of care and outcomes statewide. Maternal mortality review
committees (MMRCs) provide recommendations for preventing future pregnancy-related
deaths, including those attributable to HDP, and often collaborate with PQCs to translate
MMRC recommendations into clinical and health systems interventions. Health communication
campaigns increase awareness of urgent warning signs of HDP that indicate need for
immediate care.§§§§§§ Strategies to address health inequities in HDP include addressing
implicit, institutional, and structural racism, disparate access to clinical care,
social determinants of health, and engagement of community partners (
2
).
The findings in this report are subject to at least four limitations. First, identification
of delivery hospitalizations and HDP is dependent upon accurate ICD-10-CM coding.
Less severe cases of HDP might not be coded. In this study, approximately 4% of HDP
was documented as unspecified maternal hypertension, which precludes accurate documentation
of HDP type. Second, deaths identified using discharge disposition might underestimate
deaths during delivery hospitalization.¶¶¶¶¶¶ These data do not represent the universe
of pregnancy-related deaths, such as those that occur preceding or after delivery
hospitalizations.******* This study did not assign cause of death but instead examined
the proportion of in-hospital deaths occurring during delivery hospitalization with
an HDP diagnosis code documented. Third, CDC was unable to identify persons who delivered
more than once during the study period; the unit of analysis is delivery hospitalization.
Finally, small sample sizes did not permit the disaggregation of deaths attributable
to less frequent types of HDP and other maternal characteristics.
The prevalence of HDP increased during the 3-year study period with noted racial and
ethnic, sociodemographic, and place-based disparities. Severe HDP-associated maternal
complications and mortality are preventable with equitable implementation of public
health and clinical strategies. These include efforts across the life course for preventing
HDP, identifying, monitoring, and appropriately treating those with HDP with continuous
and coordinated care, increasing awareness of urgent maternal warning signs, and implementing
quality improvement initiatives to address severe hypertension.
Summary
What is already known about this topic?
Hypertensive disorders in pregnancy (HDPs) are common pregnancy complications and
leading causes of pregnancy-related death in the United States.
What is added by this report?
During 2017–2019, HDP prevalence among delivery hospitalizations increased from 13.3%
to 15.9%. The highest prevalence was among women aged 35–44 (18.0%) and 45–55 years
(31.0%), and those who were Black women (20.9%) or American Indian and Alaska Native
women (16.4%). Among deaths occurring during delivery hospitalization, 31.6% had a
diagnosis code for HDP documented.
What are the implications for public health practice?
Severe HDP–associated complications and mortality are preventable with equitable implementation
of quality improvement initiatives to recognize and promptly treat HDP and to increase
awareness of urgent maternal warning signs.
Related collections
Most cited references9
- Record: found
- Abstract: found
- Article: found
Racial/Ethnic Disparities in Pregnancy-Related Deaths — United States, 2007–2016
Emily Petersen, Nicole Davis, David Goodman … (2019)
- Record: found
- Abstract: found
- Article: not found
Reducing Disparities in Severe Maternal Morbidity and Mortality
ELIZABETH A. HOWELL (2018)
- Record: found
- Abstract: found
- Article: not found
Hypertension in Pregnancy: Diagnosis, Blood Pressure Goals, and Pharmacotherapy: A Scientific Statement From the American Heart Association
Vesna D. Garovic, Ralf Dechend, Thomas Easterling … (2021)