- Record: found
- Abstract: found
- Article: found
Maternal obesity increases the risk of metabolic disease and impacts renal health in offspring
Read this article at
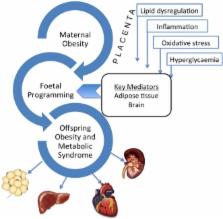
Abstract
Obesity, together with insulin resistance, promotes multiple metabolic abnormalities and is strongly associated with an increased risk of chronic disease including type 2 diabetes (T2D), hypertension, cardiovascular disease, non-alcoholic fatty liver disease (NAFLD) and chronic kidney disease (CKD). The incidence of obesity continues to rise in astronomical proportions throughout the world and affects all the different stages of the lifespan. Importantly, the proportion of women of reproductive age who are overweight or obese is increasing at an alarming rate and has potential ramifications for offspring health and disease risk. Evidence suggests a strong link between the intrauterine environment and disease programming. The current review will describe the importance of the intrauterine environment in the development of metabolic disease, including kidney disease. It will detail the known mechanisms of fetal programming, including the role of epigenetic modulation. The evidence for the role of maternal obesity in the developmental programming of CKD is derived mostly from our rodent models which will be described. The clinical implication of such findings will also be discussed.
Related collections
Most cited references98
- Record: found
- Abstract: found
- Article: not found
Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates.
- Record: found
- Abstract: found
- Article: not found
The pathogenesis of diabetic nephropathy.
- Record: found
- Abstract: found
- Article: not found