- Record: found
- Abstract: found
- Article: found
Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping
Read this article at
Abstract
The F-actin–stabilizing protein EPLIN is a mechanosensitive regulator of adherens junction remodeling in epithelial cells.
Abstract
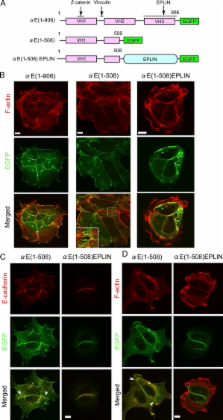
The zonula adherens (ZA), a type of adherens junction (AJ), plays a major role in epithelial cell–cell adhesions. It remains unknown how the ZA is remodeled during epithelial reorganization. Here we found that the ZA was converted to another type of AJ with punctate morphology (pAJ) at the margins of epithelial colonies. The F-actin–stabilizing protein EPLIN (epithelial protein lost in neoplasm), which functions to maintain the ZA via its association with αE-catenin, was lost in the pAJs. Consistently, a fusion of αE-catenin and EPLIN contributed to the formation of ZA but not pAJs. We show that junctional tension was important for retaining EPLIN at AJs, and another force derived from actin fibers laterally attached to the pAJs inhibited EPLIN–AJ association. Vinculin was required for general AJ formation, and it cooperated with EPLIN to maintain the ZA. These findings suggest that epithelial cells remodel their junctional architecture by responding to mechanical forces, and the αE-catenin–bound EPLIN acts as a mechanosensitive regulator for this process.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
alpha-Catenin as a tension transducer that induces adherens junction development.
- Record: found
- Abstract: found
- Article: not found
Deconstructing the cadherin-catenin-actin complex.
- Record: found
- Abstract: found
- Article: not found