- Record: found
- Abstract: found
- Article: found
A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice
Read this article at
Abstract
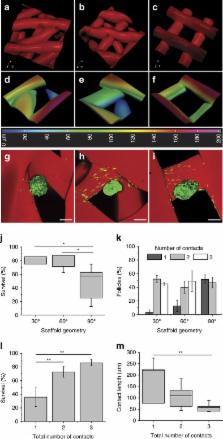
Emerging additive manufacturing techniques enable investigation of the effects of pore geometry on cell behavior and function. Here, we 3D print microporous hydrogel scaffolds to test how varying pore geometry, accomplished by manipulating the advancing angle between printed layers, affects the survival of ovarian follicles. 30° and 60° scaffolds provide corners that surround follicles on multiple sides while 90° scaffolds have an open porosity that limits follicle–scaffold interaction. As the amount of scaffold interaction increases, follicle spreading is limited and survival increases. Follicle-seeded scaffolds become highly vascularized and ovarian function is fully restored when implanted in surgically sterilized mice. Moreover, pups are born through natural mating and thrive through maternal lactation. These findings present an in vivo functional ovarian implant designed with 3D printing, and indicate that scaffold pore architecture is a critical variable in additively manufactured scaffold design for functional tissue engineering.
Abstract
There is a clinical need to develop a bioengineering system to support ovary transplantation. Here, the authors generate a bioprosthetic ovary using 3D printed scaffolds of varying pore architectures to support follicle survival and ovarian function in sterilized mice.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Three-dimensional bioprinting of thick vascularized tissues.
- Record: found
- Abstract: found
- Article: not found
Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels.
- Record: found
- Abstract: found
- Article: not found