- Record: found
- Abstract: found
- Article: found
Treatment of Brain AVMs (TOBAS): study protocol for a pragmatic randomized controlled trial
Read this article at
Abstract
Background
The management of unruptured brain arteriovenous malformation (AVM) patients remains controversial. Furthermore, curative attempts to treat ruptured AVM patients have not been questioned so far, and there is a lack of prospective data on clinical results according to treatment modality. Endovascular treatment is often used aiming to improve the safety or efficacy of surgery or radiation therapy, but benefits have never been documented in a trial. A care trial context is needed to evaluate interventions at the same time they are practised.
Methods/Trial design
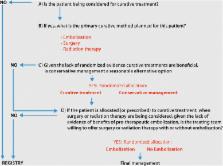
TOBAS is a pragmatic, prospective, multicenter, randomized, controlled trial and registry which offers a care trial context for brain AVM patients, including surgical resection, radiosurgery or endovascular embolization, alone or combined. The study includes two RCTs and a multimodality prospective registry. The objectives of the proposed study are to assess whether preventive interventions (surgery, embolization, radiation therapy, alone or combined), selected by the local treatment team and performed as locally practiced, randomly allocated and compared with conservative management, in unruptured brain AVM patients eligible for active or conservative management, can improve the proportion of patients having an independent outcome (modified Rankin Scale (mRS) < 3, as assessed by a standardized questionnaire administered by non-masked care personnel) at 10 years. All patients judged ineligible for randomized allocation are to be entered in a multimodal registry. The objective of a nested trial in patients with ruptured or unruptured AVMs to be treated by surgery or radiation therapy, is to assess whether pre-surgical or pre-radiation embolization, randomly allocated and compared with no embolization, can improve the proportion of patients with complete eradication of the AVM, as locally adjudicated, combined with a good clinical outcome (mRS < 3). The study will require up to 2000 patients in approximately 30 centers or more, followed for 10 years. TOBAS is registered at clinicaltrials.gov: NCT02098252 as of 25 March 2014.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
A proposed grading system for arteriovenous malformations.
- Record: found
- Abstract: found
- Article: not found
Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial.
- Record: found
- Abstract: found
- Article: not found