- Record: found
- Abstract: found
- Article: found
Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study
Read this article at
Abstract
Purpose
Prior work suggests a threshold of four courses/year of systemic corticosteroid (SCS) therapy is associated with adverse consequences. The objective of this study was to investigate the onset of adverse outcomes beginning at SCS initiation in a broad asthma population.
Patients and methods
This historical matched cohort study utilized anonymized, longitudinal medical record data (1984–2017) of patients (≥18 years) with active asthma. Matched patients with first SCS prescription (SCS arm) and no SCS exposure (non-SCS arm) were followed until first outcome event. Associations between time-varying exposure measures and onset of 17 SCS-associated adverse outcomes were estimated using Cox proportional hazard regression, adjusting for confounders, in separate models.
Results
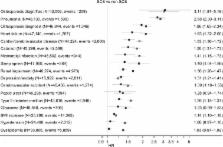
We matched 24,117 pairs of patients with median record availability before SCS initiation of 9.9 and 8.7 years and median follow-up 7.4 and 6.4 years in SCS and non-SCS arms, respectively. Compared with patients in the non-SCS arm, patients prescribed SCS had significantly increased risk of osteoporosis/osteoporotic fracture (adjusted hazard ratio 3.11; 95% CI 1.87–5.19), pneumonia (2.68; 2.30–3.11), cardio-/cerebrovascular diseases (1.53; 1.36–1.72), cataract (1.50; 1.31–1.73), sleep apnea (1.40; 1.04–1.86), renal impairment (1.36; 1.26–1.47), depression/anxiety (1.31; 1.21–1.41), type 2 diabetes (1.26; 1.15–1.37), and weight gain (1.14; 1.10–1.18). A dose-response relationship for cumulative SCS exposure with most adverse outcomes began at cumulative exposures of 1.0–<2.5 g and for some outcomes at cumulative exposures of only 0.5–<1 g (vs >0–<0.5 g reference), equivalent to four lifetime SCS courses.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
The impact of confounder selection criteria on effect estimation.
- Record: found
- Abstract: found
- Article: not found