- Record: found
- Abstract: found
- Article: found
SGLT2 inhibition restrains thyroid cancer growth via G1/S phase transition arrest and apoptosis mediated by DNA damage response signaling pathways
Read this article at
Abstract
Background
Although the prognosis for most patients with papillary thyroid cancer (PTC) is good, the present treatment is ineffective for 5–10% patients. Several studies found sodium–glucose cotransporter 2 (SGLT2) inhibitors may inhibit the growth of tumors. However, whether SGLT2 inhibitors have therapeutic effect on thyroid cancer remains unclear.
Materials and methods
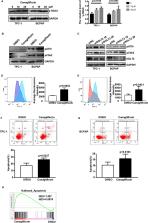
The levels of SGLT2 in PTC and normal thyroid tissue were assessed by immunohistochemistry and clinical dataset analysis. Cell growth was detected by the CCK-8 and colony formation. Glucose uptake into thyroid cancer cell was evaluated by 2-DG uptake assay. Glycolysis were analyzed by Seahorse XF Extracellular Flux Analysis. RNA-seq were used to screen differentially expressed genes of cells treated with/without canagliflozin (a SGLT2 inhibitor). Furthermore, flow cytometry, western blot, and gene set enrichment analysis were employed to elucidate cell cycle, apoptosis and the underlying mechanism of the anticancer effect of canagliflozin. The effect of canagliflozin on thyroid cancer growth was further confirmed in vivo through xenograft formation assay.
Results
SGLT2 inhibition attenuated the growth of thyroid cancer cells in vitro and in vivo. Canagliflozin inhibited glucose uptake, glycolysis and AKT/mTOR signaling activation, and increased AMPK activation in thyroid cancer cell. Furthermore, canagliflozin inhibited G1/S phase transition and cyclin D1, cyclin D3, cyclin E1, cyclin E2, and E2F1 expression levels in thyroid cancer cell. In addition, canagliflozin increased apoptosis of thyroid cancer cell. Further investigation revealed that canagliflozin could increase γ-H2AX expression levels and DNA damage response signaling ATM/CHK2 activation. In thyroid cancer patients, SGLT2 was increased in thyroid cancer and positively related to cyclin D3.
Conclusions
SGLT2 inhibition may limit glucose uptake resulting in energetic crisis, following oxidative stress mediated DNA damage and cell cycle arrest, which resulted to the increased cell apoptosis and decreased proliferation of thyroid cancer cells, suggesting a potential use for SGLT2 inhibitors as thyroid cancer therapeutics.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Understanding the Warburg effect: the metabolic requirements of cell proliferation.
- Record: found
- Abstract: found
- Article: not found
DNA damage and the balance between survival and death in cancer biology.

- Record: found
- Abstract: found
- Article: found