- Record: found
- Abstract: found
- Article: found
The Activity of SN33638, an Inhibitor of AKR1C3, on Testosterone and 17β-Estradiol Production and Function in Castration-Resistant Prostate Cancer and ER-Positive Breast Cancer
Read this article at
Abstract
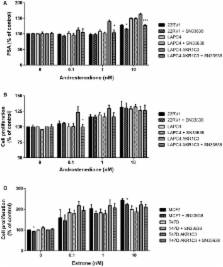
AKR1C3 is a novel therapeutic target in castration-resistant prostate cancer (CRPC) and estrogen receptor (ER)-positive breast cancer because of its ability to produce testosterone and 17β-estradiol intratumorally, thus promoting nuclear receptor signaling and tumor progression. A panel of CRPC, ER-positive breast cancer and high/low AKR1C3-expressing cell lines were treated with SN33638, a selective inhibitor of AKR1C3, in the presence of hormone or prostaglandin (PG) precursors, prior to evaluation of cell proliferation and levels of 11β-PG F 2α (11β-PGF 2α), testosterone, 17β-estradiol, and prostate-specific antigen (PSA). A meta-analysis of AKR1C3 mRNA expression in patient samples was also conducted, which revealed that AKR1C3 mRNA was upregulated in CRPC, but downregulated in ER-positive breast cancer. 11β-PGF 2α and testosterone levels in the cell line panel correlated with AKR1C3 protein expression. SN33638 prevented 11β-PGF 2α formation in cell lines that expressed AKR1C3, but partially inhibited testosterone formation and subsequently cell proliferation and/or PSA expression only in high (LAPC4 AKR1C3-overexpressing cells) or moderate (22RV1) AKR1C3-expressing cell lines. SN33638 had little effect on 17β-estradiol production or estrone-stimulated cell proliferation in ER-positive breast cancer cell lines. Although SN33638 could prevent 11β-PGF 2α formation, its ability to prevent testosterone and 17β-estradiol production and their roles in CRPC and ER-positive breast cancer progression was limited due to AKR1C3-independent steroid hormone production, except in LAPC4 AKR1C3 cells where the majority of testosterone was AKR1C3-dependent. These results suggest that inhibition of AKR1C3 is unlikely to produce therapeutic benefit in CRPC and ER-positive breast cancer patients, except possibly in the small subpopulation of CRPC patients with tumors that have upregulated AKR1C3 expression and are dependent on AKR1C3 to produce the testosterone required for their growth.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth.
- Record: found
- Abstract: found
- Article: not found
Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer.
- Record: found
- Abstract: found
- Article: not found