- Record: found
- Abstract: found
- Article: found
Dual Roles for Membrane Association of Drosophila Axin in Wnt Signaling
Read this article at
Abstract
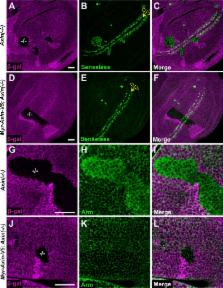
Deregulation of the Wnt signal transduction pathway underlies numerous congenital disorders and cancers. Axin, a concentration-limiting scaffold protein, facilitates assembly of a “destruction complex” that prevents signaling in the unstimulated state and a plasma membrane-associated “signalosome” that activates signaling following Wnt stimulation. In the classical model, Axin is cytoplasmic under basal conditions, but relocates to the cell membrane after Wnt exposure; however, due to the very low levels of endogenous Axin, this model is based largely on examination of Axin at supraphysiological levels. Here, we analyze the subcellular distribution of endogenous Drosophila Axin in vivo and find that a pool of Axin localizes to cell membrane proximal puncta even in the absence of Wnt stimulation. Axin localization in these puncta is dependent on the destruction complex component Adenomatous polyposis coli (Apc). In the unstimulated state, the membrane association of Axin increases its Tankyrase-dependent ADP-ribosylation and consequent proteasomal degradation to control its basal levels. Furthermore, Wnt stimulation does not result in a bulk redistribution of Axin from cytoplasmic to membrane pools, but causes an initial increase of Axin in both of these pools, with concomitant changes in two post-translational modifications, followed by Axin proteolysis hours later. Finally, the ADP-ribosylated Axin that increases rapidly following Wnt stimulation is membrane associated. We conclude that even in the unstimulated state, a pool of Axin forms membrane-proximal puncta that are dependent on Apc, and that membrane association regulates both Axin levels and Axin’s role in the rapid activation of signaling that follows Wnt exposure.
Author Summary
Axin is a scaffold protein with essential roles in Wnt signal transduction. In the classical model, the transition from the unstimulated to stimulated state is thought to be achieved by recruitment of Axin from cytosol to plasma membrane. We find that a pool of endogenous Drosophila Axin is localized in puncta juxtaposed with the cell membrane even under basal conditions and is targeted for degradation by the ADP-ribose polymerase Tankyrase. Wnt stimulation initially results in increased Axin levels in both the cytosolic and membrane pools, which may enhance the robust activation of signaling.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling.
- Record: found
- Abstract: found
- Article: not found
Analysis of genetic mosaics in developing and adult Drosophila tissues.
- Record: found
- Abstract: found
- Article: not found