- Record: found
- Abstract: found
- Article: found
Prediction of causative genes in inherited retinal disorder from fundus photography and autofluorescence imaging using deep learning techniques

Abstract
Background/Aims
To investigate the utility of a data-driven deep learning approach in patients with inherited retinal disorder (IRD) and to predict the causative genes based on fundus photography and fundus autofluorescence (FAF) imaging.
Methods
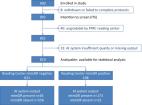
Clinical and genetic data from 1302 subjects from 729 genetically confirmed families with IRD registered with the Japan Eye Genetics Consortium were reviewed. Three categories of genetic diagnosis were selected, based on the high prevalence of their causative genes: Stargardt disease ( ABCA4), retinitis pigmentosa ( EYS) and occult macular dystrophy ( RP1L1). Fundus photographs and FAF images were cropped in a standardised manner with a macro algorithm. Images for training/testing were selected using a randomised, fourfold cross-validation method. The application program interface was established to reach the learning accuracy of concordance (target: >80%) between the genetic diagnosis and the machine diagnosis ( ABCA4, EYS, RP1L1 and normal).
Results
A total of 417 images from 156 Japanese subjects were examined, including 115 genetically confirmed patients caused by the three prevalent causative genes and 41 normal subjects. The mean overall test accuracy for fundus photographs and FAF images was 88.2% and 81.3%, respectively. The mean overall sensitivity/specificity values for fundus photographs and FAF images were 88.3%/97.4% and 81.8%/95.5%, respectively.
Conclusion
A novel application of deep neural networks in the prediction of the causative IRD genes from fundus photographs and FAF, with a high prediction accuracy of over 80%, was highlighted. These achievements will extensively promote the quality of medical care by facilitating early diagnosis, especially by non-specialists, access to care, reducing the cost of referrals, and preventing unnecessary clinical and genetic testing.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Dermatologist-level classification of skin cancer with deep neural networks
- Record: found
- Abstract: found
- Article: found
Clinically applicable deep learning for diagnosis and referral in retinal disease
- Record: found
- Abstract: found
- Article: found