- Record: found
- Abstract: found
- Article: found
Autoantibodies to Posttranslational Modifications in Rheumatoid Arthritis
Read this article at
Abstract
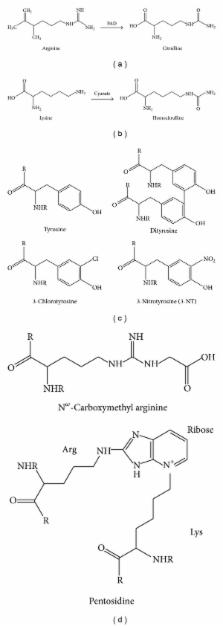
Autoantibodies have been associated with human pathologies for a long time, particularly with autoimmune diseases (AIDs). Rheumatoid factor (RF) is known since the late 1930s to be associated with rheumatoid arthritis (RA). The discovery of anticitrullinated protein antibodies in the last century has changed this and other posttranslational modifications (PTM) relevant to RA have since been described. Such PTM introduce neoepitopes in proteins that can generate novel autoantibody specificities. The recent recognition of these novel specificities in RA provides a unique opportunity to understand human B-cell development in vivo. In this paper, we will review the three of the main classes of PTMs already associated with RA: citrullination, carbamylation, and oxidation. With the advancement of research methodologies it should be expected that other autoantibodies against PTM proteins could be discovered in patients with autoimmune diseases. Many of such autoantibodies may provide significant biomarker potential.
Related collections
Most cited references275
- Record: found
- Abstract: found
- Article: not found
The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide.
- Record: found
- Abstract: found
- Article: not found
Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage.
- Record: found
- Abstract: found
- Article: not found