- Record: found
- Abstract: found
- Article: found
Growth signaling promotes chronological aging in budding yeast by inducing superoxide anions that inhibit quiescence
Read this article at
Abstract
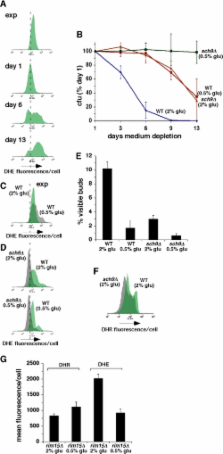
Inhibition of growth signaling pathways protects against aging and age-related diseases in parallel with reduced oxidative stress. The relationships between growth signaling, oxidative stress and aging remain unclear. Here we report that in Saccharomyces cerevisiae, alterations in growth signaling pathways impact levels of superoxide anions that promote chronological aging and inhibit growth arrest of stationary phase cells in G0/G1. Factors that decrease intracellular superoxide anions in parallel with enhanced longevity and more efficient G0/G1 arrest include genetic inactivation of growth signaling pathways that inhibit Rim15p, which activates oxidative stress responses, and downregulation of these pathways by caloric restriction. Caloric restriction also reduces superoxide anions independently of Rim15p by elevating levels of H 2O 2, which activates superoxide dismutases. In contrast, high glucose or mutations that activate growth signaling accelerate chronological aging in parallel with increased superoxide anions and reduced efficiency of stationary phase G0/G1 arrest. High glucose also activates DNA damage responses and preferentially kills stationary phase cells that fail to arrest growth in G0/G1. These findings suggest that growth signaling promotes chronological aging in budding yeast by elevating superoxide anions that inhibit quiescence and induce DNA replication stress. A similar mechanism likely contributes to aging and age-related diseases in complex eukaryotes.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
AMP-activated protein kinase induces a p53-dependent metabolic checkpoint.
- Record: found
- Abstract: found
- Article: not found
Living on a break: cellular senescence as a DNA-damage response.
- Record: found
- Abstract: found
- Article: not found