- Record: found
- Abstract: found
- Article: found
Functional morphology of the blood-brain barrier in health and disease
Read this article at
Abstract
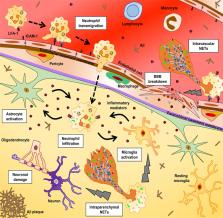
The adult quiescent blood–brain barrier (BBB), a structure organised by endothelial cells through interactions with pericytes, astrocytes, neurons and microglia in the neurovascular unit, is highly regulated but fragile at the same time. In the past decade, there has been considerable progress in understanding not only the molecular pathways involved in BBB development, but also BBB breakdown in neurological diseases. Specifically, the Wnt/β-catenin, retinoic acid and sonic hedgehog pathways moved into the focus of BBB research. Moreover, angiopoietin/Tie2 signalling that is linked to angiogenic processes has gained attention in the BBB field. Blood vessels play an essential role in initiation and progression of many diseases, including inflammation outside the central nervous system (CNS). Therefore, the potential influence of CNS blood vessels in neurological diseases associated with BBB alterations or neuroinflammation has become a major focus of current research to understand their contribution to pathogenesis. Moreover, the BBB remains a major obstacle to pharmaceutical intervention in the CNS. The complications may either be expressed by inadequate therapeutic delivery like in brain tumours, or by poor delivery of the drug across the BBB and ineffective bioavailability. In this review, we initially describe the cellular and molecular components that contribute to the steady state of the healthy BBB. We then discuss BBB alterations in ischaemic stroke, primary and metastatic brain tumour, chronic inflammation and Alzheimer’s disease. Throughout the review, we highlight common mechanisms of BBB abnormalities among these diseases, in particular the contribution of neuroinflammation to BBB dysfunction and disease progression, and emphasise unique aspects of BBB alteration in certain diseases such as brain tumours. Moreover, this review highlights novel strategies to monitor BBB function by non-invasive imaging techniques focussing on ischaemic stroke, as well as novel ways to modulate BBB permeability and function to promote treatment of brain tumours, inflammation and Alzheimer’s disease. In conclusion, a deep understanding of signals that maintain the healthy BBB and promote fluctuations in BBB permeability in disease states will be key to elucidate disease mechanisms and to identify potential targets for diagnostics and therapeutic modulation of the BBB.
Related collections
Most cited references101
- Record: found
- Abstract: found
- Article: not found
Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation
- Record: found
- Abstract: found
- Article: not found
Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo.
- Record: found
- Abstract: found
- Article: not found