- Record: found
- Abstract: found
- Article: found
Simultaneous production of laccase and degradation of bisphenol A with Trametes versicolor cultivated on agricultural wastes
Read this article at
Abstract
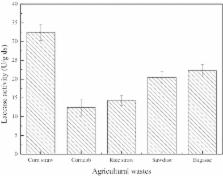
Solid state fermentation with Trametes versicolor was carried out on agricultural wastes containing bisphenol A (BPA). It was found that BPA degradation was along with the occurrence of laccase production, and wheat bran and corn straw were identified as suitable mixed substrates for laccase production. In the process of BPA degradation with T. versicolor, laccase activity increased rapidly at the 6th–10th day after inoculation. Moreover, BPA can enhance the production of laccase. After 10 days of fermentation, degradation rate of BPA exceeded 90% without the usage of mediators ABTS and acetosyringone at pH 4.0–8.0. In addition, metal ions did not affect the BPA degradation with T. versicolor. In vitro, the optimum pH range of BPA degradation with laccase was in the acidic region with the optimal performance of pH 5.0. Metal ions Cu 2+, Zn 2+, and Co 2+ showed little effect on BPA degradation. However, Fe 3+ and Fe 2+ substantially inhibited the BPA degradation. Natural mediator acetosyringone showed optimum enhancement on BPA degradation. Greater than 90% of the estrogenic activity of BPA was removed by T. versicolor and its laccase. Compared to in vitro degradation with laccase, this study shows that the process of simultaneous laccase production and BPA degradation with T. versicolor was more advantageous since BPA can enhance the laccase production, mediators were unnecessary, degradation rate was not affected by metal ions, and the applicable pH range was broader. This study concludes that T. versicolor and laccase have great potential to treat industrial wastewater containing BPA.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
A review of the environmental fate, effects, and exposures of bisphenol A.
- Record: found
- Abstract: found
- Article: not found
Laccase: new functions for an old enzyme
- Record: found
- Abstract: found
- Article: not found