- Record: found
- Abstract: found
- Article: found
Biomarkers for Alzheimer’s Disease (AD) and the Application of Precision Medicine
Read this article at
Abstract
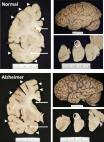
An accurate diagnosis of Alzheimer’s disease (AD) currently stands as one of the most difficult and challenging in all of clinical neurology. AD is typically diagnosed using an integrated knowledge and assessment of multiple biomarkers and interrelated factors. These include the patient’s age, gender and lifestyle, medical and genetic history (both clinical- and family-derived), cognitive, physical, behavioral and geriatric assessment, laboratory examination of multiple AD patient biofluids, especially within the systemic circulation (blood serum) and cerebrospinal fluid (CSF), multiple neuroimaging-modalities of the brain’s limbic system and/or retina, followed up in many cases by post-mortem neuropathological examination to finally corroborate the diagnosis. More often than not, prospective AD cases are accompanied by other progressive, age-related dementing neuropathologies including, predominantly, a neurovascular and/or cardiovascular component, multiple-infarct dementia (MID), frontotemporal dementia (FTD) and/or strokes or ‘mini-strokes’ often integrated with other age-related neurological and non-neurological disorders including cardiovascular disease and cancer. Especially over the last 40 years, enormous research efforts have been undertaken to discover, characterize, and quantify more effectual and reliable biological markers for AD, especially during the pre-clinical or prodromal stages of AD so that pre-emptive therapeutic treatment strategies may be initiated. While a wealth of genetic, neurobiological, neurochemical, neuropathological, neuroimaging and other diagnostic information obtainable for a single AD patient can be immense: ( i) it is currently challenging to integrate and formulate a definitive diagnosis for AD from this multifaceted and multidimensional information; and ( ii) these data are unfortunately not directly comparable with the etiopathological patterns of other AD patients even when carefully matched for age, gender, familial genetics, and drug history. Four decades of AD research have repeatedly indicated that diagnostic profiles for AD are reflective of an extremely heterogeneous neurological disorder. This commentary will illuminate the heterogeneity of biomarkers for AD, comment on emerging investigative approaches and discuss why ‘precision medicine’ is emerging as our best paradigm yet for the most accurate and definitive prediction, diagnosis, and prognosis of this insidious and lethal brain disorder.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: found
The neuropathological diagnosis of Alzheimer’s disease
- Record: found
- Abstract: found
- Article: not found
Diagnosis and Management of Dementia: Review
- Record: found
- Abstract: found
- Article: not found