- Record: found
- Abstract: found
- Article: found
Umbelliferone instability during an analysis involving its extraction process

Read this article at
Abstract
Abstract
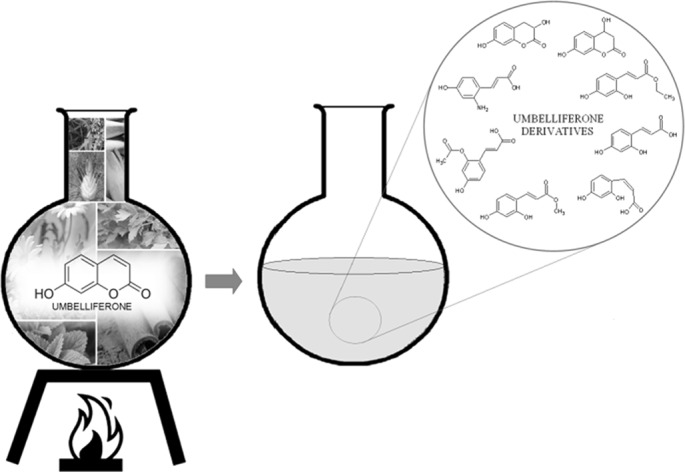
Umbelliferone (7-hydroxycoumarin) is one of the most popular compounds of the coumarins family. This compound receives the attention of scientists due to its diverse bioactivities in a number of applications in various therapeutic fields. An interesting aspect of umbelliferone is its structural lability. The enzymatic degradation process of umbelliferone to its hydroxylated (esculetin), glucosylated (skimmin), and methylated (herniarin) derivatives is already known from the literature. In this paper, we describe the possibility of umbelliferone transformation to other derivatives. We found that eight compounds were formed from umbelliferone during its simulated extraction under reflux performed in different conditions (different heating times and solvents used). Six of them (4,7-dihydroxy-3,4-dihydro-2 H-chromen-2-one, 3,7-dihydroxy-3,4-dihydro-2 H-chromen-2-one, methyl (2 E)-3-(2,4-dihydroxyphenyl)prop-2-enoate, ethyl (2 E)-3-(2,4-dihydroxyphenyl)prop-2-enoate, (2 E)-3-[2-(acetyloxy)-4-hydroxyphenyl]prop-2-enoic acid, (2 E)-3-(2-amino-4-hydroxyphenyl)prop-2-enoic acid) have not been reported yet. Some of these compounds were also identified in extracts of plant materials containing umbelliferone—chamomile flower and cinnamon bark. Compound separation was carried out using the HPLC apparatus. All compounds were identified based on their MS fragmentation paths. The presented results are useful for food producers and consumers, as umbelliferone transformation products can be formed during food product storage, their preparation or processing.
Related collections
Most cited references7
- Record: found
- Abstract: not found
- Article: not found
Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes
- Record: found
- Abstract: found
- Article: not found
Accumulation of coumarins in Arabidopsis thaliana.
- Record: found
- Abstract: found
- Article: not found