- Record: found
- Abstract: found
- Article: found
ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease
Read this article at
Abstract
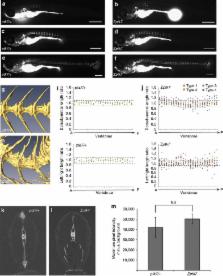
Scoliosis is a complex genetic disorder of the musculoskeletal system, characterized by three-dimensional rotation of the spine. Curvatures caused by malformed vertebrae (congenital scoliosis (CS)) are apparent at birth. Spinal curvatures with no underlying vertebral abnormality (idiopathic scoliosis (IS)) most commonly manifest during adolescence. The genetic and biological mechanisms responsible for IS remain poorly understood due largely to limited experimental models. Here we describe zygotic ptk7 (Z ptk7) mutant zebrafish, deficient in a critical regulator of Wnt signalling, as the first genetically defined developmental model of IS. We identify a novel sequence variant within a single IS patient that disrupts PTK7 function, consistent with a role for dysregulated Wnt activity in disease pathogenesis. Furthermore, we demonstrate that embryonic loss-of-gene function in maternal-zygotic ptk7 mutants (MZ ptk7) leads to vertebral anomalies associated with CS. Our data suggest novel molecular origins of, and genetic links between, congenital and idiopathic forms of disease.
Abstract
 Scoliosis is a complex genetic disorder characterized by spinal curvature. Here, the
authors present experimental zebrafish models of idiopathic and congenital scoliosis
and suggest a role for dysregulated Wnt activity in scoliosis aetiology.
Scoliosis is a complex genetic disorder characterized by spinal curvature. Here, the
authors present experimental zebrafish models of idiopathic and congenital scoliosis
and suggest a role for dysregulated Wnt activity in scoliosis aetiology.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
A molecular mechanism for the effect of lithium on development.
- Record: found
- Abstract: found
- Article: not found
Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis.
- Record: found
- Abstract: found
- Article: not found