- Record: found
- Abstract: found
- Article: not found
Retrospective Multicenter Cohort Study Shows that Early Interferon Therapy is Associated with Favorable Clinical Responses in COVID-19 Patients

Read this article at
Summary
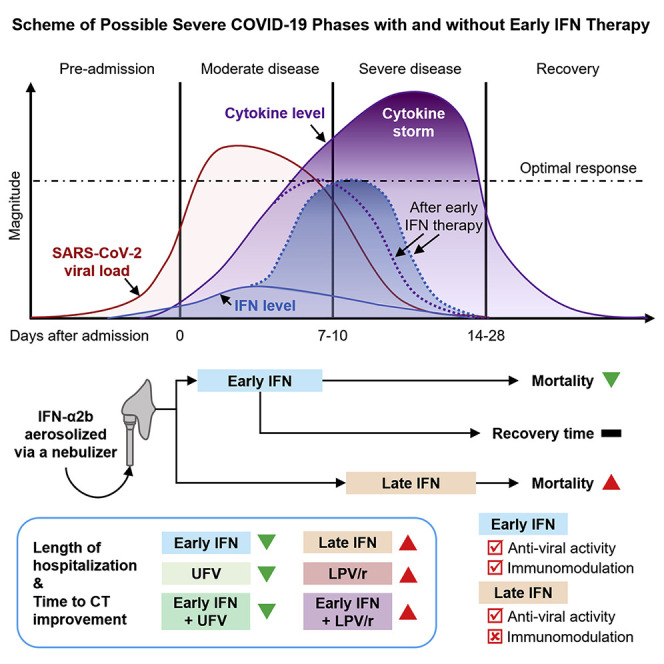
Interferons (IFN) are widely used in treating coronavirus disease 2019 (COVID-19) patients. However, recent report of ACE2, the host factor mediating SARS-Cov-2 infection, as interferon-stimulated, raised considerable safety concern. To examine the association between the use and timing of IFN-α2b and clinical outcomes, we analyzed in a retrospective multicenter cohort study 446 COVID-19 patients in Hubei, China. Regression models estimated that early administration (≤5 days after admission) of IFN-α2b was associated with reduced in-hospital mortality compared to no IFN-α2b, while late administration of IFN-α2b was associated with increased mortality. Among survivors, early IFN-α2b was not associated with hospital discharge or CT scan improvement, while late IFN-α2b was associated with delayed recovery. Additionally, early IFN-α2b and umifenovir (UFV) alone or together were associated with reduced mortality and accelerated recovery compared to lopinavir/ritonavir (LPV/r) alone. We concluded that administration of IFN-α2b during the early stage of COVID-19 may induce favorable clinical responses.
Graphical Abstract
Highlights
-
•
242 of 446 analyzed COVID-19 patients received interferon-α2b, a type I interferon.
-
•
Early initiation of interferon therapy was associated with reduced mortality.
-
•
Interferon therapy was not associated with recovery time for COVID-19.
-
•
Interferon-α2b was associated with better responses than lopinavir/ritonavir.
Abstract
In a retrospective cohort study of 446 COVID-19 patients, Wang et al. determine that early administration of interferon-α2b was associated with reduced in-hospital mortality. In contrast, late interferon therapy increased mortality and delayed recovery, suggesting the timing of interferon therapy is crucial for favorable responses in COVID-19 patients.