- Record: found
- Abstract: found
- Article: found
Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension
Read this article at
Abstract
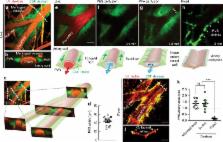
Flow of cerebrospinal fluid (CSF) through perivascular spaces (PVSs) in the brain is important for clearance of metabolic waste. Arterial pulsations are thought to drive flow, but this has never been quantitatively shown. We used particle tracking to quantify CSF flow velocities in PVSs of live mice. CSF flow is pulsatile and driven primarily by the cardiac cycle. The speed of the arterial wall matches that of the CSF, suggesting arterial wall motion is the principal driving mechanism, via a process known as perivascular pumping. Increasing blood pressure leaves the artery diameter unchanged but changes the pulsations of the arterial wall, increasing backflow and thereby reducing net flow in the PVS. Perfusion-fixation alters the normal flow direction and causes a 10-fold reduction in PVS size. We conclude that particle tracking velocimetry enables the study of CSF flow in unprecedented detail and that studying the PVS in vivo avoids fixation artifacts.
Abstract
Arterial pulsations are thought to drive CSF flow through perivascular spaces (PVSs), but this has never been quantitatively shown. Using particle tracking to quantify CSF flow velocities in PVSs of live mice, the authors show that flow speeds match the instantaneous speeds of the pulsing artery walls that form the inner boundaries of the PVSs.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β.
- Record: found
- Abstract: not found
- Article: not found
2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults
- Record: found
- Abstract: found
- Article: not found