- Record: found
- Abstract: found
- Article: found
Silicified collagen scaffold induces semaphorin 3A secretion by sensory nerves to improve in-situ bone regeneration

Read this article at
Abstract
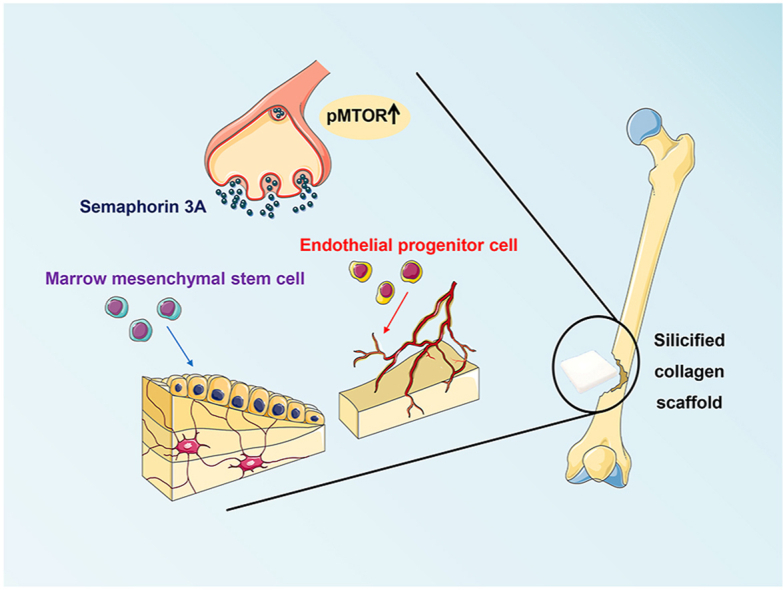
Sensory nerves promote osteogenesis through the release of neuropeptides. However, the potential application and mechanism in which sensory nerves promote healing of bone defects in the presence of biomaterials remain elusive. The present study identified that new bone formation was more abundantly produced after implantation of silicified collagen scaffolds into defects created in the distal femur of rats. The wound sites were accompanied by extensive nerve innervation and angiogenesis. Sensory nerve dysfunction by capsaicin injection resulted in significant inhibition of silicon-induced osteogenesis in the aforementioned rodent model. Application of extracellular silicon in vitro induced axon outgrowth and increased expression of semaphorin 3 A (Sema3A) and semaphorin 4D (Sema4D) in the dorsal root ganglion (DRG), as detected by the upregulation of signaling molecules. Culture medium derived from silicon-stimulated DRG cells promoted proliferation and differentiation of bone marrow mesenchymal stem cells and endothelial progenitor cells. These effects were inhibited by the use of Sema3A neutralizing antibodies but not by Sema4D neutralizing antibodies. Knockdown of Sema3A in DRG blocked silicon-induced osteogenesis and angiogenesis almost completely in a femoral defect rat model, whereas overexpression of Sema3A promoted the silicon-induced phenomena. Activation of “mechanistic target of rapamycin” (mTOR) pathway and increase of Sema3A production were identified in the DRG of rats that were implanted with silicified collagen scaffolds. These findings support the role of silicon in inducing Sema3A production by sensory nerves, which, in turn, stimulates osteogenesis and angiogenesis. Taken together, silicon has therapeutic potential in orthopedic rehabilitation.
Graphical abstract
Highlights
-
•
Nerve innervation, vascularization and tissue mineralization integrated into a single scaffold.
-
•
Silicified collagen scaffolds has therapeutic potential in orthopedic rehabilitation.
-
•
Silicified collagen scaffolds promote in-situ bone regeneration via sensory nerve innervation and semaphorin 3A production.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
mTOR Signaling in Growth, Metabolism, and Disease.
- Record: found
- Abstract: found
- Article: not found
Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone.
- Record: found
- Abstract: found
- Article: not found