- Record: found
- Abstract: found
- Article: found
Characterization of the ExoU activation mechanism using EPR and integrative modeling
Read this article at
Abstract
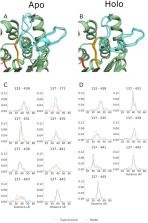
ExoU, a type III secreted phospholipase effector of Pseudomonas aeruginosa, serves as a prototype to model large, dynamic, membrane-associated proteins. ExoU is synergistically activated by interactions with membrane lipids and ubiquitin. To dissect the activation mechanism, structural homology was used to identify an unstructured loop of approximately 20 residues in the ExoU amino acid sequence. Mutational analyses indicate the importance of specific loop amino acid residues in mediating catalytic activity. Engineered disulfide cross-links show that loop movement is required for activation. Site directed spin labeling EPR and DEER (double electron–electron resonance) studies of apo and holo states demonstrate local conformational changes at specific sites within the loop and a conformational shift of the loop during activation. These data are consistent with the formation of a substrate-binding pocket providing access to the catalytic site. DEER distance distributions were used as constraints in RosettaDEER to construct ensemble models of the loop in both apo and holo states, significantly extending the range for modeling a conformationally dynamic loop.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules.
- Record: found
- Abstract: not found
- Article: not found
THE DISTRIBUTION OF THE FLORA IN THE ALPINE ZONE.1
- Record: found
- Abstract: found
- Article: not found