- Record: found
- Abstract: found
- Article: found
Repetitive transcranial magnetic stimulation (rTMS) in autism spectrum disorder: protocol for a multicentre randomised controlled clinical trial
Read this article at
Abstract
Introduction
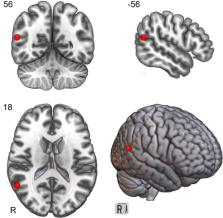
There are no well-established biomedical treatments for the core symptoms of autism spectrum disorder (ASD). A small number of studies suggest that repetitive transcranial magnetic stimulation (rTMS), a non-invasive brain stimulation technique, may improve clinical and cognitive outcomes in ASD. We describe here the protocol for a funded multicentre randomised controlled clinical trial to investigate whether a course of rTMS to the right temporoparietal junction (rTPJ), which has demonstrated abnormal brain activation in ASD, can improve social communication in adolescents and young adults with ASD.
Methods and analysis
This study will evaluate the safety and efficacy of a 4-week course of intermittent theta burst stimulation (iTBS, a variant of rTMS) in ASD. Participants meeting criteria for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition ASD (n=150, aged 14–40 years) will receive 20 sessions of either active iTBS (600 pulses) or sham iTBS (in which a sham coil mimics the sensation of iTBS, but no active stimulation is delivered) to the rTPJ. Participants will undergo a range of clinical, cognitive, epi/genetic, and neurophysiological assessments before and at multiple time points up to 6 months after iTBS. Safety will be assessed via a structured questionnaire and adverse event reporting. The study will be conducted from November 2020 to October 2024.
Ethics and dissemination
The study was approved by the Human Research Ethics Committee of Monash Health (Melbourne, Australia) under Australia’s National Mutual Acceptance scheme. The trial will be conducted according to Good Clinical Practice, and findings will be written up for scholarly publication.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
The REDCap consortium: Building an international community of software platform partners
- Record: found
- Abstract: found
- Article: found
Identification of common genetic risk variants for autism spectrum disorder
- Record: found
- Abstract: found
- Article: not found