- Record: found
- Abstract: found
- Article: found
Polyadenylation-Dependent Control of Long Noncoding RNA Expression by the Poly(A)-Binding Protein Nuclear 1
Read this article at
Abstract
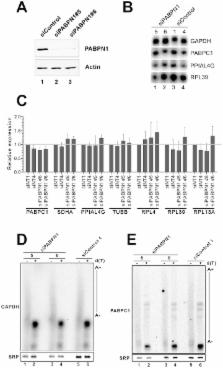
The poly(A)-binding protein nuclear 1 (PABPN1) is a ubiquitously expressed protein that is thought to function during mRNA poly(A) tail synthesis in the nucleus. Despite the predicted role of PABPN1 in mRNA polyadenylation, little is known about the impact of PABPN1 deficiency on human gene expression. Specifically, it remains unclear whether PABPN1 is required for general mRNA expression or for the regulation of specific transcripts. Using RNA sequencing (RNA–seq), we show here that the large majority of protein-coding genes express normal levels of mRNA in PABPN1–deficient cells, arguing that PABPN1 may not be required for the bulk of mRNA expression. Unexpectedly, and contrary to the view that PABPN1 functions exclusively at protein-coding genes, we identified a class of PABPN1–sensitive long noncoding RNAs (lncRNAs), the majority of which accumulated in conditions of PABPN1 deficiency. Using the spliced transcript produced from a snoRNA host gene as a model lncRNA, we show that PABPN1 promotes lncRNA turnover via a polyadenylation-dependent mechanism. PABPN1–sensitive lncRNAs are targeted by the exosome and the RNA helicase MTR4/SKIV2L2; yet, the polyadenylation activity of TRF4-2, a putative human TRAMP subunit, appears to be dispensable for PABPN1–dependent regulation. In addition to identifying a novel function for PABPN1 in lncRNA turnover, our results provide new insights into the post-transcriptional regulation of human lncRNAs.
Author Summary
In eukaryotic cells, protein-coding genes are transcribed to produce pre-messenger RNAs (pre–mRNAs) that are processed at the 3′ end by the addition of a sequence of poly-adenosine. This 3′ end poly(A) tail normally confers positive roles to the mRNA life cycle by stimulating nuclear export and translation. The fundamental role of mRNA polyadenylation is generally mediated by the activity of poly(A)-binding proteins (PABPs) that bind to the 3′ poly(A) tail of eukaryotic mRNAs. In the nucleus, the evolutionarily conserved poly(A)-binding protein PABPN1 is thought to be important for gene expression, as it stimulates mRNA polyadenylation in biochemical assays. Using a high-throughput sequencing approach that quantitatively measures the level of RNA expressed from all genes, we addressed the global impact of a PABPN1 deficiency on human gene expression. Notably, we found that most mRNAs were normally expressed in PABPN1–deficient cells, a result inconsistent with a role for PABPN1 in general mRNA metabolism. Surprisingly, our genome-wide analysis unveiled a new function for PABPN1 in a polyadenylation-dependent pathway of RNA decay that targets non-protein coding genes. Our discovery that PABPN1 functions in the regulation of noncoding RNAs raises the possibility that oculopharyngeal muscular dystrophy, a disease associated with mutations in the PABPN1 gene, is caused by defective expression of noncoding RNAs.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Long noncoding RNA as modular scaffold of histone modification complexes.
- Record: found
- Abstract: found
- Article: not found
Ab initio reconstruction of transcriptomes of pluripotent and lineage committed cells reveals gene structures of thousands of lincRNAs
- Record: found
- Abstract: found
- Article: not found