- Record: found
- Abstract: found
- Article: found
Linking oxidative and reductive clusters to prepare crystalline porous catalysts for photocatalytic CO 2 reduction with H 2O
Read this article at
Abstract
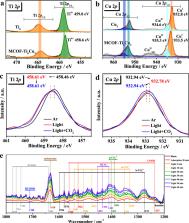
Mimicking natural photosynthesis to convert CO 2 with H 2O into value-added fuels achieving overall reaction is a promising way to reduce the atmospheric CO 2 level. Casting the catalyst of two or more catalytic sites with rapid electron transfer and interaction may be an effective strategy for coupling photocatalytic CO 2 reduction and H 2O oxidation. Herein, based on the MOF ∪ COF collaboration, we have carefully designed and synthesized a crystalline hetero-metallic cluster catalyst denoted MCOF-Ti 6Cu 3 with spatial separation and functional cooperation between oxidative and reductive clusters. It utilizes dynamic covalent bonds between clusters to promote photo-induced charge separation and transfer efficiency, to drive both the photocatalytic oxidative and reductive reactions. MCOF-Ti 6Cu 3 exhibits fine activity in the conversion of CO 2 with water into HCOOH (169.8 μmol g −1h −1). Remarkably, experiments and theoretical calculations reveal that photo-excited electrons are transferred from Ti to Cu, indicating that the Cu cluster is the catalytic reduction center.
Abstract
A crystalline hetero-metallic cluster catalyst based on a covalent organic framework strategy is reported. The catalyst can facilitate both photocatalytic oxidative and reductive reactions leading to efficient production of HCOOH from CO2 and H2O.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Covalent organic frameworks comprising cobalt porphyrins for catalytic CO₂ reduction in water.
- Record: found
- Abstract: not found
- Article: not found
Artificial photosynthesis for solar water-splitting
- Record: found
- Abstract: found
- Article: not found