- Record: found
- Abstract: found
- Article: found
CD82 hypomethylation is essential for tuberculosis pathogenesis via regulation of RUNX1-Rab5/22
Read this article at
Abstract
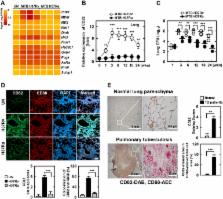
The tumor suppressor gene CD82/KAI1 is a member of the tetraspanin superfamily and organizes various membrane-based processes. Mycobacterium tuberculosis (MTB) persists in host macrophages by interfering with phagolysosome biogenesis and inflammatory responses, but the role of CD82 in controlling the intracellular survival of pathogenic mycobacteria within macrophages remains poorly understood. In this study, we demonstrated that the virulent MTB strain H37Rv (MTB Rv) induced CD82 promoter hypomethylation, resulting in CD82 expression. Targeting of the runt-related transcription factor 1 (RUNX1) by CD82 is essential for phagosome arrest via interacting with Rab5/22. This arrest is required for the intracellular growth of MTB in vitro and in vivo, but not for that of MTB H37Ra (MTB Ra) in macrophages. In addition, knockdown or knockout of CD82 or RUNX1 increased antibacterial host defense via phagolysosome biogenesis, inflammatory cytokine production, and subsequent antimicrobial activity both in vitro and in vivo. Notably, the levels of CD82 and RUNX1 in granulomas were elevated in tuberculosis (TB) patients, indicating that CD82 and RUNX1 have clinical significance in human TB. Our findings identify a previously unrecognized role of CD82 hypomethylation in the regulation of phagosome maturation, enhanced intracellular survival, and the innate host immune response to MTB. Thus, the CD82–RUNX1–Rab5/22 axis may be a previously unrecognized virulence mechanism of MTB pathogenesis.
Tuberculosis: Evading host defences
The tuberculosis-causing bacterium Mycobacterium tuberculosis regulates a tumor suppressor gene in order to survive and grow in host immune cells. Chul-Su Yang and colleagues at Hanyang University, South Korea, have found that the bacterium can stimulate the expression of CD82 in macrophages by removing methyl groups from its DNA sequence. CD82’s hypomethylated region interacts with and activates proteins that interfere with the cell’s ability to mount an inflammatory response and degrade bacteria in specialized intracellular vesicles called lysosomes. The increased survival rate of CD82-deficient mice following infection with tuberculosis and the elevated levels of CD82 protein found in the inflammatory lesions of patients with tuberculosis further support a previously unrecognized role for this protein in M. tuberculosis infection. Targeting CD82-mediated signaling could be a promising approach for designing new therapeutics.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
PROMO: detection of known transcription regulatory elements using species-tailored searches.
- Record: found
- Abstract: found
- Article: not found
Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development.
- Record: found
- Abstract: found
- Article: not found