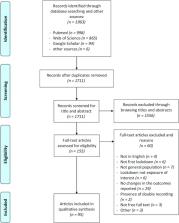
Introduction Systematic reviews and meta-analyses have become increasingly important in health care. Clinicians read them to keep up to date with their field [1],[2], and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research [3], and some health care journals are moving in this direction [4]. As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers' ability to assess the strengths and weaknesses of those reviews. Several early studies evaluated the quality of review reports. In 1987, Mulrow examined 50 review articles published in four leading medical journals in 1985 and 1986 and found that none met all eight explicit scientific criteria, such as a quality assessment of included studies [5]. In 1987, Sacks and colleagues [6] evaluated the adequacy of reporting of 83 meta-analyses on 23 characteristics in six domains. Reporting was generally poor; between one and 14 characteristics were adequately reported (mean = 7.7; standard deviation = 2.7). A 1996 update of this study found little improvement [7]. In 1996, to address the suboptimal reporting of meta-analyses, an international group developed a guidance called the QUOROM Statement (QUality Of Reporting Of Meta-analyses), which focused on the reporting of meta-analyses of randomized controlled trials [8]. In this article, we summarize a revision of these guidelines, renamed PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses), which have been updated to address several conceptual and practical advances in the science of systematic reviews (Box 1). Box 1: Conceptual Issues in the Evolution from QUOROM to PRISMA Completing a Systematic Review Is an Iterative Process The conduct of a systematic review depends heavily on the scope and quality of included studies: thus systematic reviewers may need to modify their original review protocol during its conduct. Any systematic review reporting guideline should recommend that such changes can be reported and explained without suggesting that they are inappropriate. The PRISMA Statement (Items 5, 11, 16, and 23) acknowledges this iterative process. Aside from Cochrane reviews, all of which should have a protocol, only about 10% of systematic reviewers report working from a protocol [22]. Without a protocol that is publicly accessible, it is difficult to judge between appropriate and inappropriate modifications. Conduct and Reporting Research Are Distinct Concepts This distinction is, however, less straightforward for systematic reviews than for assessments of the reporting of an individual study, because the reporting and conduct of systematic reviews are, by nature, closely intertwined. For example, the failure of a systematic review to report the assessment of the risk of bias in included studies may be seen as a marker of poor conduct, given the importance of this activity in the systematic review process [37]. Study-Level Versus Outcome-Level Assessment of Risk of Bias For studies included in a systematic review, a thorough assessment of the risk of bias requires both a “study-level” assessment (e.g., adequacy of allocation concealment) and, for some features, a newer approach called “outcome-level” assessment. An outcome-level assessment involves evaluating the reliability and validity of the data for each important outcome by determining the methods used to assess them in each individual study [38]. The quality of evidence may differ across outcomes, even within a study, such as between a primary efficacy outcome, which is likely to be very carefully and systematically measured, and the assessment of serious harms [39], which may rely on spontaneous reports by investigators. This information should be reported to allow an explicit assessment of the extent to which an estimate of effect is correct [38]. Importance of Reporting Biases Different types of reporting biases may hamper the conduct and interpretation of systematic reviews. Selective reporting of complete studies (e.g., publication bias) [28] as well as the more recently empirically demonstrated “outcome reporting bias” within individual studies [40],[41] should be considered by authors when conducting a systematic review and reporting its results. Though the implications of these biases on the conduct and reporting of systematic reviews themselves are unclear, some previous research has identified that selective outcome reporting may occur also in the context of systematic reviews [42]. Terminology The terminology used to describe a systematic review and meta-analysis has evolved over time. One reason for changing the name from QUOROM to PRISMA was the desire to encompass both systematic reviews and meta-analyses. We have adopted the definitions used by the Cochrane Collaboration [9]. A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research, and to collect and analyze data from the studies that are included in the review. Statistical methods (meta-analysis) may or may not be used to analyze and summarize the results of the included studies. Meta-analysis refers to the use of statistical techniques in a systematic review to integrate the results of included studies. Developing the PRISMA Statement A three-day meeting was held in Ottawa, Canada, in June 2005 with 29 participants, including review authors, methodologists, clinicians, medical editors, and a consumer. The objective of the Ottawa meeting was to revise and expand the QUOROM checklist and flow diagram, as needed. The executive committee completed the following tasks, prior to the meeting: a systematic review of studies examining the quality of reporting of systematic reviews, and a comprehensive literature search to identify methodological and other articles that might inform the meeting, especially in relation to modifying checklist items. An international survey of review authors, consumers, and groups commissioning or using systematic reviews and meta-analyses was completed, including the International Network of Agencies for Health Technology Assessment (INAHTA) and the Guidelines International Network (GIN). The survey aimed to ascertain views of QUOROM, including the merits of the existing checklist items. The results of these activities were presented during the meeting and are summarized on the PRISMA Web site (http://www.prisma-statement.org/). Only items deemed essential were retained or added to the checklist. Some additional items are nevertheless desirable, and review authors should include these, if relevant [10]. For example, it is useful to indicate whether the systematic review is an update [11] of a previous review, and to describe any changes in procedures from those described in the original protocol. Shortly after the meeting a draft of the PRISMA checklist was circulated to the group, including those invited to the meeting but unable to attend. A disposition file was created containing comments and revisions from each respondent, and the checklist was subsequently revised 11 times. The group approved the checklist, flow diagram, and this summary paper. Although no direct evidence was found to support retaining or adding some items, evidence from other domains was believed to be relevant. For example, Item 5 asks authors to provide registration information about the systematic review, including a registration number, if available. Although systematic review registration is not yet widely available [12],[13], the participating journals of the International Committee of Medical Journal Editors (ICMJE) [14] now require all clinical trials to be registered in an effort to increase transparency and accountability [15]. Those aspects are also likely to benefit systematic reviewers, possibly reducing the risk of an excessive number of reviews addressing the same question [16],[17] and providing greater transparency when updating systematic reviews. The PRISMA Statement The PRISMA Statement consists of a 27-item checklist (Table 1; see also Text S1 for a downloadable Word template for researchers to re-use) and a four-phase flow diagram (Figure 1; see also Figure S1 for a downloadable Word template for researchers to re-use). The aim of the PRISMA Statement is to help authors improve the reporting of systematic reviews and meta-analyses. We have focused on randomized trials, but PRISMA can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. PRISMA may also be useful for critical appraisal of published systematic reviews. However, the PRISMA checklist is not a quality assessment instrument to gauge the quality of a systematic review. 10.1371/journal.pmed.1000097.g001 Figure 1 Flow of information through the different phases of a systematic review. 10.1371/journal.pmed.1000097.t001 Table 1 Checklist of items to include when reporting a systematic review or meta-analysis. Section/Topic # Checklist Item Reported on Page # TITLE Title 1 Identify the report as a systematic review, meta-analysis, or both. ABSTRACT Structured summary 2 Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. INTRODUCTION Rationale 3 Describe the rationale for the review in the context of what is already known. Objectives 4 Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). METHODS Protocol and registration 5 Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. Eligibility criteria 6 Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. Information sources 7 Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. Search 8 Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. Study selection 9 State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). Data collection process 10 Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. Data items 11 List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. Risk of bias in individual studies 12 Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. Summary measures 13 State the principal summary measures (e.g., risk ratio, difference in means). Synthesis of results 14 Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. Risk of bias across studies 15 Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). Additional analyses 16 Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. RESULTS Study selection 17 Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. Study characteristics 18 For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. Risk of bias within studies 19 Present data on risk of bias of each study and, if available, any outcome-level assessment (see Item 12). Results of individual studies 20 For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot. Synthesis of results 21 Present results of each meta-analysis done, including confidence intervals and measures of consistency. Risk of bias across studies 22 Present results of any assessment of risk of bias across studies (see Item 15). Additional analysis 23 Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). DISCUSSION Summary of evidence 24 Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., health care providers, users, and policy makers). Limitations 25 Discuss limitations at study and outcome level (e.g., risk of bias), and at review level (e.g., incomplete retrieval of identified research, reporting bias). Conclusions 26 Provide a general interpretation of the results in the context of other evidence, and implications for future research. FUNDING Funding 27 Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. From QUOROM to PRISMA The new PRISMA checklist differs in several respects from the QUOROM checklist, and the substantive specific changes are highlighted in Table 2. Generally, the PRISMA checklist “decouples” several items present in the QUOROM checklist and, where applicable, several checklist items are linked to improve consistency across the systematic review report. 10.1371/journal.pmed.1000097.t002 Table 2 Substantive specific changes between the QUOROM checklist and the PRISMA checklist (a tick indicates the presence of the topic in QUOROM or PRISMA). Section/Topic Item QUOROM PRISMA Comment Abstract √ √ QUOROM and PRISMA ask authors to report an abstract. However, PRISMA is not specific about format. Introduction Objective √ This new item (4) addresses the explicit question the review addresses using the PICO reporting system (which describes the participants, interventions, comparisons, and outcome(s) of the systematic review), together with the specification of the type of study design (PICOS); the item is linked to Items 6, 11, and 18 of the checklist. Methods Protocol √ This new item (5) asks authors to report whether the review has a protocol and if so how it can be accessed. Methods Search √ √ Although reporting the search is present in both QUOROM and PRISMA checklists, PRISMA asks authors to provide a full description of at least one electronic search strategy (Item 8). Without such information it is impossible to repeat the authors' search. Methods Assessment of risk of bias in included studies √ √ Renamed from “quality assessment” in QUOROM. This item (12) is linked with reporting this information in the results (Item 19). The new concept of “outcome-level” assessment has been introduced. Methods Assessment of risk of bias across studies √ This new item (15) asks authors to describe any assessments of risk of bias in the review, such as selective reporting within the included studies. This item is linked with reporting this information in the results (Item 22). Discussion √ √ Although both QUOROM and PRISMA checklists address the discussion section, PRISMA devotes three items (24–26) to the discussion. In PRISMA the main types of limitations are explicitly stated and their discussion required. Funding √ This new item (27) asks authors to provide information on any sources of funding for the systematic review. The flow diagram has also been modified. Before including studies and providing reasons for excluding others, the review team must first search the literature. This search results in records. Once these records have been screened and eligibility criteria applied, a smaller number of articles will remain. The number of included articles might be smaller (or larger) than the number of studies, because articles may report on multiple studies and results from a particular study may be published in several articles. To capture this information, the PRISMA flow diagram now requests information on these phases of the review process. Endorsement The PRISMA Statement should replace the QUOROM Statement for those journals that have endorsed QUOROM. We hope that other journals will support PRISMA; they can do so by registering on the PRISMA Web site. To underscore to authors, and others, the importance of transparent reporting of systematic reviews, we encourage supporting journals to reference the PRISMA Statement and include the PRISMA Web address in their Instructions to Authors. We also invite editorial organizations to consider endorsing PRISMA and encourage authors to adhere to its principles. The PRISMA Explanation and Elaboration Paper In addition to the PRISMA Statement, a supporting Explanation and Elaboration document has been produced [18] following the style used for other reporting guidelines [19]–[21]. The process of completing this document included developing a large database of exemplars to highlight how best to report each checklist item, and identifying a comprehensive evidence base to support the inclusion of each checklist item. The Explanation and Elaboration document was completed after several face to face meetings and numerous iterations among several meeting participants, after which it was shared with the whole group for additional revisions and final approval. Finally, the group formed a dissemination subcommittee to help disseminate and implement PRISMA. Discussion The quality of reporting of systematic reviews is still not optimal [22]–[27]. In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias [22], even though there is overwhelming evidence both for its existence [28] and its impact on the results of systematic reviews [29]. Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately [30]. Although the absence of reporting such an assessment does not necessarily indicate that it was not done, reporting an assessment of possible publication bias is likely to be a marker of the thoroughness of the conduct of the systematic review. Several approaches have been developed to conduct systematic reviews on a broader array of questions. For example, systematic reviews are now conducted to investigate cost-effectiveness [31], diagnostic [32] or prognostic questions [33], genetic associations [34], and policy making [35]. The general concepts and topics covered by PRISMA are all relevant to any systematic review, not just those whose objective is to summarize the benefits and harms of a health care intervention. However, some modifications of the checklist items or flow diagram will be necessary in particular circumstances. For example, assessing the risk of bias is a key concept, but the items used to assess this in a diagnostic review are likely to focus on issues such as the spectrum of patients and the verification of disease status, which differ from reviews of interventions. The flow diagram will also need adjustments when reporting individual patient data meta-analysis [36]. We have developed an explanatory document [18] to increase the usefulness of PRISMA. For each checklist item, this document contains an example of good reporting, a rationale for its inclusion, and supporting evidence, including references, whenever possible. We believe this document will also serve as a useful resource for those teaching systematic review methodology. We encourage journals to include reference to the explanatory document in their Instructions to Authors. Like any evidence-based endeavor, PRISMA is a living document. To this end we invite readers to comment on the revised version, particularly the new checklist and flow diagram, through the PRISMA Web site. We will use such information to inform PRISMA's continued development. Supporting Information Figure S1 Flow of information through the different phases of a systematic review (downloadable template document for researchers to re-use). (0.08 MB DOC) Click here for additional data file. Text S1 Checklist of items to include when reporting a systematic review or meta-analysis (downloadable template document for researchers to re-use). (0.04 MB DOC) Click here for additional data file.