- Record: found
- Abstract: found
- Article: found
Activation of metabotropic glutamate receptor 1 regulates hippocampal CA1 region excitability in rats with status epilepticus by suppressing the HCN1 channel
Read this article at
Abstract

Abstract
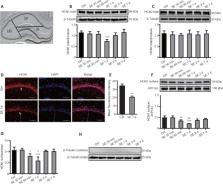
Dysregulation of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels alters neuronal excitability. However, the role of HCN channels in status epilepticus is not fully understood. In this study, we established rat models of pentylenetetrazole-induced status epilepticus. We performed western blot assays and immunofluorescence staining. Our results showed that HCN1 channel protein expression, particularly HCN1 surface protein, was significantly decreased in the hippocampal CA1 region, whereas the expression of HCN2 channel protein was unchanged. Moreover, metabolic glutamate receptor 1 (mGluR1) protein expression was increased after status epilepticus. The mGluR1 agonist (RS)-3,5-dihydroxyphenylglycine injected intracerebroventricularly increased the sensitivity and severity of pentylenetetrazole-induced status epilepticus, whereas application of the mGluR1 antagonist (+)-2-methyl-4-carboxyphenylglycine (LY367385) alleviated the severity of pentylenetetrazole-induced status epilepticus. The results from double immunofluorescence labeling revealed that mGluR1 and HCN1 were co-localized in the CA1 region. Subsequently, a protein kinase A inhibitor (H89) administered intraperitoneally successfully reversed HCN1 channel inhibition, thereby suppressing the severity and prolonging the latency of pentylenetetrazole-induced status epilepticus. Furthermore, H89 reduced the level of mGluR1, downregulated cyclic adenosine monophosphate (cAMP)/protein kinase A expression, significantly increased tetratricopeptide repeat-containing Rab8b-interacting protein (TRIP8b) (1a-4) expression, and restored TRIP8b (1b-2) levels. TRIP8b (1a-4) and TRIP8b (1b-2) are subunits of Rab8b interacting protein that regulate HCN1 surface protein.
Related collections
Most cited references104
- Record: found
- Abstract: found
- Article: found
Animal models of necrotizing enterocolitis: review of the literature and state of the art
- Record: found
- Abstract: not found
- Article: not found
Modification of seizure activity by electrical stimulation: II. Motor seizure
- Record: found
- Abstract: found
- Article: not found