- Record: found
- Abstract: found
- Article: found
Brain-Gut-Microbiota Axis in Alzheimer’s Disease
Read this article at
Abstract
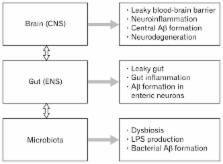
Disturbances along the brain-gut-microbiota axis may significantly contribute to the pathogenesis of neurodegenerative disorders. Alzheimer’s disease (AD) is the most frequent cause of dementia characterized by a progressive decline in cognitive function associated with the formation of amyloid beta (Aβ) plaques and neurofibrillary tangles. Alterations in the gut microbiota composition induce increased permeability of the gut barrier and immune activation leading to systemic inflammation, which in turn may impair the blood-brain barrier and promote neuroinflammation, neural injury, and ultimately neurodegeneration. Recently, Aβ has also been recognized as an antimicrobial peptide participating in the innate immune response. However, in the dysregulated state, Aβ may reveal harmful properties. Importantly, bacterial amyloids through molecular mimicry may elicit cross-seeding of misfolding and induce microglial priming. The Aβ seeding and propagation may occur at different levels of the brain-gut-microbiota axis. The potential mechanisms of amyloid spreading include neuron-to-neuron or distal neuron spreading, direct blood-brain barrier crossing or via other cells as astrocytes, fibroblasts, microglia, and immune system cells. A growing body of experimental and clinical data confirms a key role of gut dysbiosis and gut microbiota-host interactions in neurodegeneration. The convergence of gut-derived inflammatory response together with aging and poor diet in the elderly contribute to the pathogenesis of AD. Modification of the gut microbiota composition by food-based therapy or by probiotic supplementation may create new preventive and therapeutic options in AD.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Amyloid beta: structure, biology and structure-based therapeutic development
- Record: found
- Abstract: found
- Article: not found
RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain.

- Record: found
- Abstract: found
- Article: found