- Record: found
- Abstract: found
- Article: found
Efficacy and safety of extended-release oxcarbazepine (Oxtellar XR™) as adjunctive therapy in patients with refractory partial-onset seizures: a randomized controlled trial
Read this article at
Abstract
Objective
To evaluate the efficacy, tolerability, and safety of once-daily 1200 mg and 2400 mg SPN-804 (Oxtellar XR™, Supernus Pharmaceuticals), an extended-release tablet formulation of oxcarbazepine (OXC), added to 1-3 concomitant antiepileptic drugs (AEDs) in adults with refractory partial-onset seizures, with or without secondary generalization.
Methods
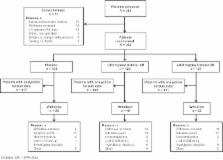
The Prospective, Randomized Study of OXC XR in Subjects with Partial Epilepsy Refractory (PROSPER) study was a multinational, randomized, double-blind, parallel-group Phase 3 study. The primary efficacy endpoint was median percent reduction from baseline in monthly (28-day) seizure frequency for the 16-week double-blind treatment period in the intent-to-treat (ITT) population with analyzable seizure data. Other efficacy analyses included proportion of patients with ≥ 50% seizure reduction, proportion of patients seizure free, and the relationship between clinical response and plasma concentration.
Results
Median percent reduction was -28.7% for placebo, −38.2% ( P = 0.08 vs placebo) for once-daily SPN-804 1200 mg, and −42.9% ( P = 0.003) for SPN-804 2400 mg. Responder rates were 28.1%, 36.1% ( P = 0.08), and 40.7% ( P = 0.02); 16-week seizure-free rates in a pragmatic ITT analysis were 3.3%, 4.9% ( P = 0.59), and 11.4% ( P = 0.008), respectively. When data were analyzed separately for study site clusters, a post hoc analysis demonstrated that both SPN-804 dosages were significantly superior to placebo in median percent seizure reduction (placebo: −13.3%; 1200 mg: −34.5%, P = 0.02; 2400 mg: −52.7%, P = 0.006) in the North American study site cluster. A concentration–response analysis also supported a clinically meaningful effect for 1200 mg. Adverse event types reflected the drug's established profile. Adverse event frequency was consistent with a pharmacokinetic profile in which SPN-804 produces lower peak plasma concentrations vs immediate-release OXC. Once-daily dosing was not associated with any new safety signals.
Related collections
Most cited references14
- Record: found
- Abstract: not found
- Article: not found
Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy.
- Record: found
- Abstract: found
- Article: not found
Adjunctive perampanel for refractory partial-onset seizures: randomized phase III study 304.
- Record: found
- Abstract: found
- Article: not found