- Record: found
- Abstract: found
- Article: not found
Alkyne Derivatives of SARS-CoV-2 Main Protease Inhibitors Including Nirmatrelvir Inhibit by Reacting Covalently with the Nucleophilic Cysteine

Read this article at
Abstract
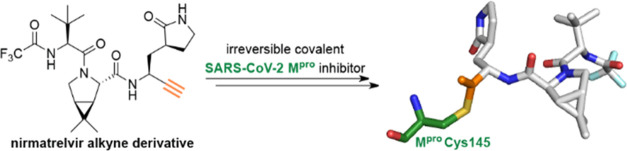
Nirmatrelvir (PF-07321332) is a nitrile-bearing small-molecule inhibitor that, in combination with ritonavir, is used to treat infections by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Nirmatrelvir interrupts the viral life cycle by inhibiting the SARS-CoV-2 main protease (M pro), which is essential for processing viral polyproteins into functional nonstructural proteins. We report studies which reveal that derivatives of nirmatrelvir and other M pro inhibitors with a nonactivated terminal alkyne group positioned similarly to the electrophilic nitrile of nirmatrelvir can efficiently inhibit isolated M pro and SARS-CoV-2 replication in cells. Mass spectrometric and crystallographic evidence shows that the alkyne derivatives inhibit M pro by apparent irreversible covalent reactions with the active site cysteine (Cys145), while the analogous nitriles react reversibly. The results highlight the potential for irreversible covalent inhibition of M pro and other nucleophilic cysteine proteases by alkynes, which, in contrast to nitriles, can be functionalized at their terminal position to optimize inhibition and selectivity, as well as pharmacodynamic and pharmacokinetic properties.
Related collections
Most cited references109

- Record: found
- Abstract: found
- Article: found
A new coronavirus associated with human respiratory disease in China

- Record: found
- Abstract: found
- Article: found
The species Severe acute respiratory syndrome-related coronavirus : classifying 2019-nCoV and naming it SARS-CoV-2
- Record: found
- Abstract: found
- Article: not found
