- Record: found
- Abstract: found
- Article: found
A long-acting LEAP2 analog reduces hepatic steatosis and inflammation and causes marked weight loss in mice

Read this article at
Abstract
Objective
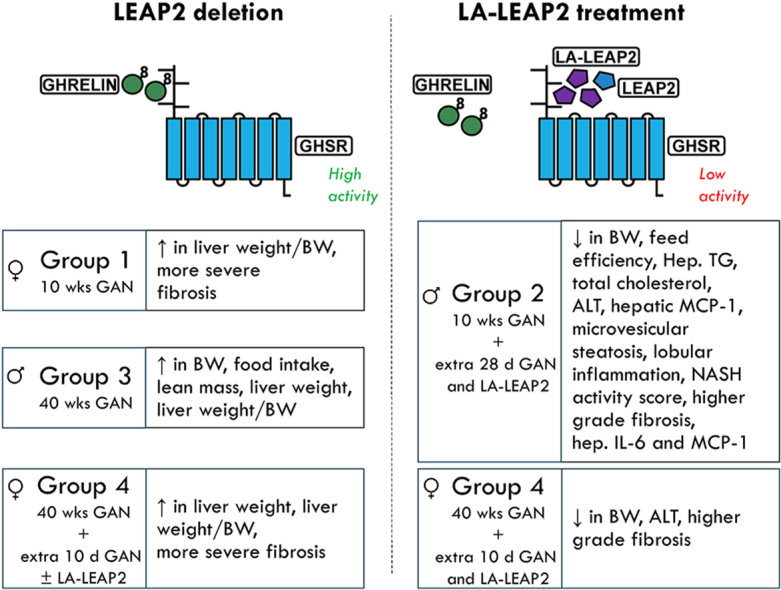
The number of individuals affected by metabolic dysfunction associated fatty liver disease [1] is on the rise, yet hormonal contributors to the condition remain incompletely described and only a single FDA-approved treatment is available. Some studies suggest that the hormones ghrelin and LEAP2, which act as agonist and antagonist/inverse agonist, respectively, for the G protein coupled receptor GHSR, may influence the development of MAFLD. For instance, ghrelin increases hepatic fat whereas synthetic GHSR antagonists do the opposite. Also, hepatic steatosis is less prominent in standard chow-fed ghrelin-KO mice but more prominent in 42% high-fat diet-fed female LEAP2-KO mice.
Methods
Here, we sought to determine the therapeutic potential of a long-acting LEAP2 analog (LA-LEAP2) to treat MAFLD in mice. LEAP2-KO and wild-type littermate mice were fed a Gubra-Amylin-NASH (GAN) diet for 10 or 40 wks, with some randomized to an additional 28 or 10 days of GAN diet, respectively, while treated with LA-LEAP2 vs Vehicle. Various metabolic parameters were followed and biochemical and histological assessments of MAFLD were made.
Results
Among the most notable metabolic effects, daily LA-LEAP2 administration to both LEAP2-KO and wild-type littermates during the final 4 wks of a 14 wk-long GAN diet challenge markedly reduced liver weight, hepatic triglycerides, plasma ALT, hepatic microvesicular steatosis, hepatic lobular inflammation, NASH activity scores, and prevalence of higher-grade fibrosis. These changes were accompanied by prominent reductions in body weight, without effects on food intake, and reduced plasma total cholesterol. Daily LA-LEAP2 administration during the final 10 d of a 41.5 wk-long GAN diet challenge also reduced body weight, plasma ALT, and plasma total cholesterol in LEAP2-KO and wild-type littermates and prevalence of higher grade fibrosis in LEAP2-KO mice.
Conclusions
Administration of LA-LEAP2 to mice fed a MAFLD-prone diet markedly improves several facets of MAFLD, including hepatic steatosis, hepatic lobular inflammation, higher-grade hepatic fibrosis, and transaminitis. These changes are accompanied by prominent reductions in body weight and lowered plasma total cholesterol. Taken together, these data suggest that LEAP2 analogs such as LA-LEAP2 hold promise for the treatment of MAFLD and obesity.
Graphical abstract
Highlights
-
•
We studied the therapeutic potential of a novel LEAP2 analog to treat MAFLD in mice.
-
•
Specifically, the LEAP2 analog was given to MAFLD-prone diet-fed LEAP2-KO & WT mice.
-
•
Administration of the LEAP2 analog markedly improved several facets of MAFLD.
-
•
Included were hepatic steatosis, inflammation, high-grade fibrosis, & transaminitis.
-
•
The LEAP2 analog also prominently reduced body weight & lowered plasma cholesterol.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Mechanisms of NAFLD development and therapeutic strategies
- Record: found
- Abstract: found
- Article: not found
MAFLD: A consensus-driven proposed nomenclature for metabolic associated fatty liver disease
- Record: found
- Abstract: found
- Article: not found