- Record: found
- Abstract: found
- Article: not found
Defect-Engineered Nanostructured Ni/MOF-Derived Carbons for an Efficient Aqueous Battery-Type Energy Storage Device

Read this article at
Abstract
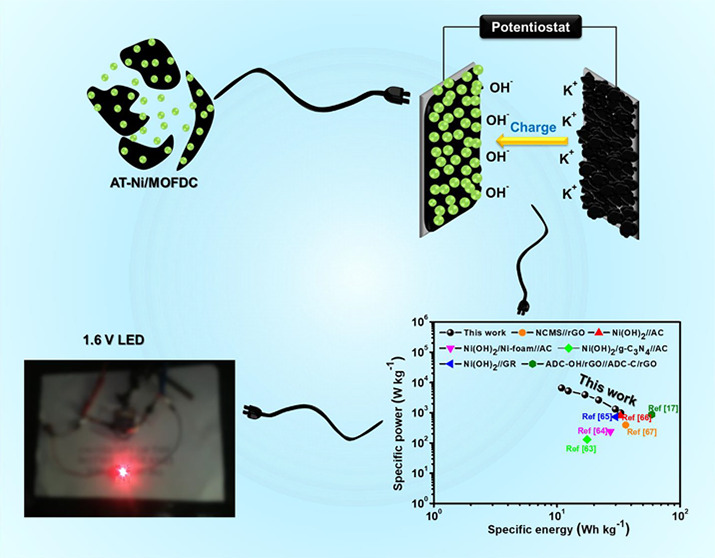
A Ni-based metal–organic framework (Ni-MOF) has been synthesized using a microwave-assisted strategy and converted to nanostructured Ni/MOF-derived mesoporous carbon (Ni/MOFDC) by carbonization and acid treatment (AT-Ni/MOFDC). The materials are well characterized with Raman, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray spectroscopy (EDX), and Brunauer–Emmett–Teller (BET), revealing that chemical etching confers on the AT-Ni/MOFDC-reduced average nanoparticle size (high surface area) and structural defects including oxygen vacancies. AT-Ni/MOFDC displays low series resistances and a higher specific capacity ( C s) of 199 mAh g –1 compared to Ni/MOFDC (92 mAh g –1). This study shows that the storage mechanism of the Ni-based electrode as a battery-type energy storage (BTES) system can be controlled by both non-faradic and faradic processes and dependent on the sweep rate or current density. AT-Ni/MOFDC reveals mixed contributions at different rates: 75.2% faradic and 24.8% non-faradic contributions at 5 mV s –1, and 34.1% faradic and 65.9% non-faradic at 50 mV s –1. The full BTES device was assembled with AT-Ni/MOFDC as the cathode and acetylene black (AB) as the anode. Compared to recent literature, the AT-Ni/MOFDC//AB BTES device exhibits high energy (33 Wh kg –1) and high power (983 W kg –1) with excellent cycling performance (about 88% capacity retention over 2000 cycles). This new finding opens a window of opportunity for the rational designing of next-generation energy storage devices, supercapatteries, that combine the characteristics of batteries (high energy) and supercapacitors (high power).
Related collections
Most cited references59
- Record: found
- Abstract: not found
- Article: not found
Pseudocapacitive oxide materials for high-rate electrochemical energy storage
- Record: found
- Abstract: not found
- Article: not found
Metal-organic frameworks (MOFs).
- Record: found
- Abstract: found
- Article: not found
