- Record: found
- Abstract: found
- Article: found
pH-Responsive Biocompatible Fluorescent Hydrogel for Selective Sensing and Adsorptive Recovery of Dysprosium

Read this article at
Abstract
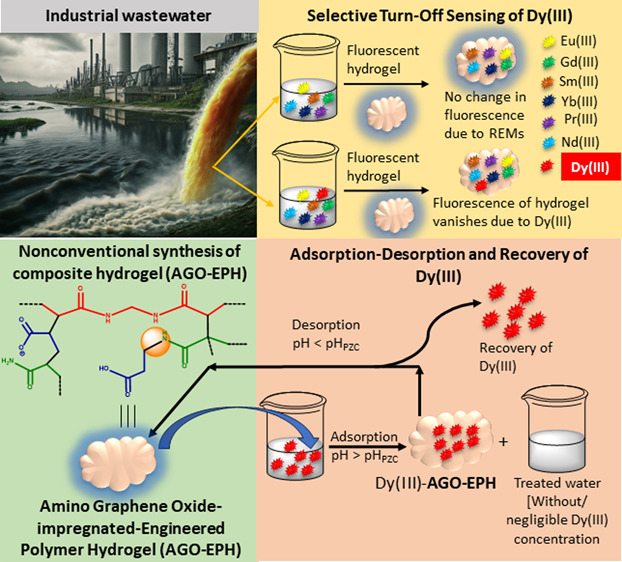
The elevated accumulation of electronic wastes, especially containing Dysprosium ion [i.e., Dy(III)], is emerging as a potential environmental threat. To overcome the deleterious effects of Dy(III), detection and removal of Dy(III) is crucial. Moreover, recovery of high-value Dy(III) is economically beneficial. However, the availability of a single material, capable of sensing Dy(III) in nanomolar concentration and simultaneously adsorbing it with high adsorption capacity (AC), is rare. Therefore, to solve this problem, a pH-responsive fluorescent amino graphene oxide-impregnated-engineered polymer hydrogel ( AGO-EPH) has been synthesized, suitable for selective sensing of Dy(III) in nanomolar concentration and adsorbing it from wastewater at ambient temperature. This terpolymeric hydrogel is synthesized from two nonfluorescent monomers, propenoic acid (PNA) and prop-2-enamide (PEAM), along with an in situ generated comonomer (3-acrylamidopropanoic acid/AAPPA) through N– H activation during polymerization. Surface properties and structural details of AGO-EPH are established using NMR, FTIR, XRD, TEM, SEM, EDX, Raman, MALDI-mass, and DLS studies. The AGO-EPH exhibits blue fluorescence with selective turn-off sensing of Dy(III) with the detection limit of 1.88 × 10 –7 (M). The maximum AC of AGO-EPH is 41.97 ± 0.39 mg g –1. The developed AGO-EPH shows consistent adsorption–desorption property over five cycles, with more than 90% desorption efficiency per cycle, confirming significant recovery of the valuable Dy(III). From Logic gate calculations, complexation of Dy(III) and AGO-EPH may be the reason behind fluorescence quenching. The AGO-EPH also shows antibacterial action against ∼3 × 10 8 cells mL –1 of E. coli solution. Overall, the developed pH-responsive engineered hydrogel can be used as a potential low-cost sensing device and reusable adsorbent for Dy(III).
Related collections
Most cited references59
- Record: found
- Abstract: not found
- Article: not found
Recycling of rare earths: a critical review
- Record: found
- Abstract: not found
- Article: not found
Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence
- Record: found
- Abstract: not found
- Article: not found