- Record: found
- Abstract: found
- Article: found
367. Safety and Immunogenicity of a 50-μg mRNA-1273 Vaccine Booster for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) in Adolescents

Read this article at
Abstract
Background
Increased community COVID-19 cases prompted the clinical evaluation of an mRNA-1273 booster dose (BD) in TeenCOVE adolescent participants (12-17 years) who received a 2-dose mRNA-1273 primary series. At ≥5 months after dose 2 (coinciding with the omicron wave peak in Jan 2022), TeenCOVE participants were offered an optional 50-µg mRNA-1273 BD. Here, we inferred BD effectiveness in adolescents by demonstrating non-inferiority (NI) of neutralizing antibody (nAb) responses post-BD vs young adults (18-25 years) post-dose 2 of 100-μg mRNA-1273 primary series in the pivotal phase 3 COVE study, where efficacy was established.
Methods
Up to 6 months post-BD, 1405 participants were monitored for COVID-19 and safety (solicited adverse reactions [≤7 days post-BD]; unsolicited AEs [≤28 days post-BD]; and medically attended, serious [SAEs], of special interest [AESI], or leading to discontinuation [throughout study]). At Day 29 post-BD, nAb geometric mean concentrations (GMCs) were measured against ancestral D614G SARS-CoV-2 spike protein. Binding antibodies (bAbs) against spike protein of ancestral strain or alpha, beta, delta, and gamma variants were measured.
Results
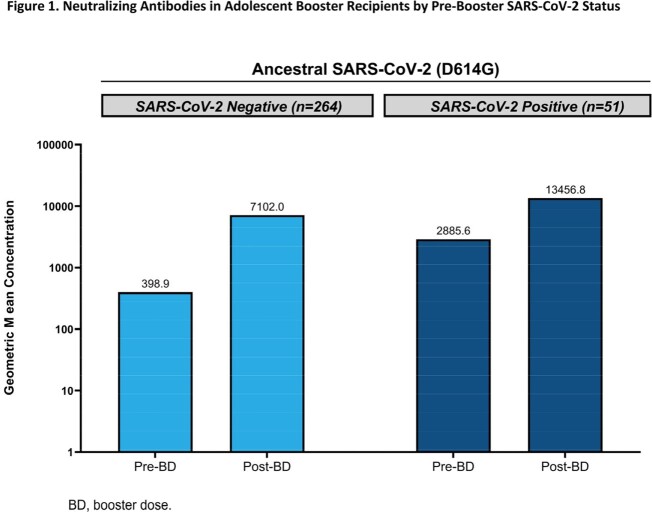
An mRNA-1273 BD was generally well-tolerated; reactogenicity profiles were consistent to the phase 3 COVE study in young adults. There were no severe COVID-19 cases, deaths, or investigator-reported vaccine-related SAEs or AESIs. In pre-booster SARS-CoV-2 negative participants, the ratio of adolescent (n=264) BD-Day 29 GMC (7102; 95% CI, 6553.2-7696.8) to young adult (n=294) Day 57 GMC (1400.4; 1272.7-1541.0) was 5.1 (4.5-5.7), meeting NI criterion for GMR ( Fig 1; Table 1). The group difference in seroresponse rate (SRR) between adolescents and young adults was 0.7% (95% CI, -0.8 to 2.4), meeting NI criterion for SRR difference ( Table 1). Robust bAb responses were observed, including against variants.
Conclusion
Effectiveness of an mRNA-1273 BD against COVID-19 in adolescents was inferred by successful immunobridging to young adults in the pivotal phase 3 trial. The benefits of variant-containing mRNA-1273 boosters demonstrated in adults is also anticipated to be conferred to adolescents. The overall benefit-risk profile of an mRNA-1273 BD is favorable in adolescents.
Disclosures
Amparo Figueroa, MD, MPH, Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Gary Berman, MD, Moderna, Inc.: Grant/Research Support Honghong Zhou, Ph.D., Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Weiping Deng, PhD, Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Monali Patel, MS, Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Bethany Girard, Ph.D., Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Anne Yeakey, MD, Moderna, Inc.: Advisor/Consultant Karen Slobod, MD, Moderna, Inc.: Advisor/Consultant Frances Priddy, MD, MPH, Moderna, Inc.: Salary|Moderna, Inc.: Stocks/Bonds Jacqueline Miller, MD, Moderna, Inc.: salary|Moderna, Inc.: Stocks/Bonds Rituparna Das, M.D., Moderna, Inc.: Salary|Moderna, Inc.: Stocks/Bonds