- Record: found
- Abstract: found
- Article: found
Palladium-Catalyzed Access to Benzocyclobutenone-Derived Ketonitrones via C(sp 2)–H Functionalization
rapid-communication

Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
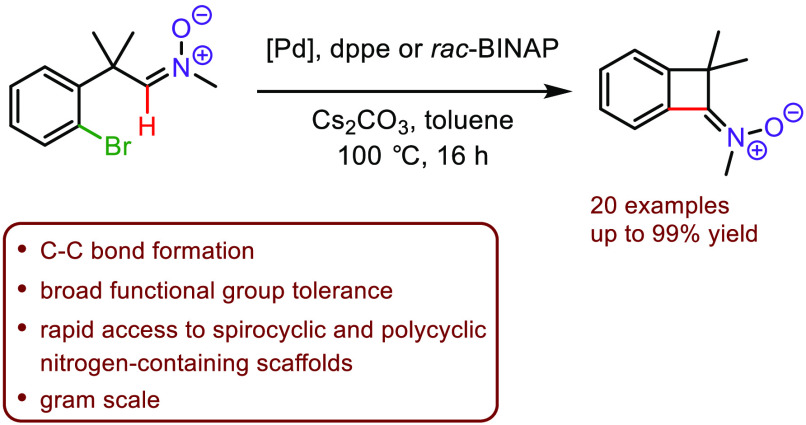
The palladium-catalyzed C(sp 2)–H functionalization of bromoaryl aldonitrones leading to benzocyclobutenone-derived ketonitrones is described. This method allows for the preparation of a wide range of strained, four-membered ketonitrones with broad functional group tolerance. Downstream transformations of the formed products were readily demonstrated, illustrating the synthetic utility of the obtained benzocyclobutenone-derived nitrones for the construction of polycyclic nitrogen-containing scaffolds.
Related collections
Most cited references63
- Record: found
- Abstract: not found
- Article: not found
Beyond Directing Groups: Transition-Metal-Catalyzed CH Activation of Simple Arenes
- Record: found
- Abstract: found
- Article: not found
Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups
Zhanxiang Liu, Yuhong Zhang, Zhengkai Chen … (2015)
- Record: found
- Abstract: found
- Article: not found
Recent Methodologies That Exploit C-C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes.
Gabriele Fumagalli, Steven Stanton, John Bower (2017)