- Record: found
- Abstract: found
- Article: found
Hydrogen storage efficiency of Fe doped carbon nanotubes: molecular simulation study†

Read this article at
Abstract
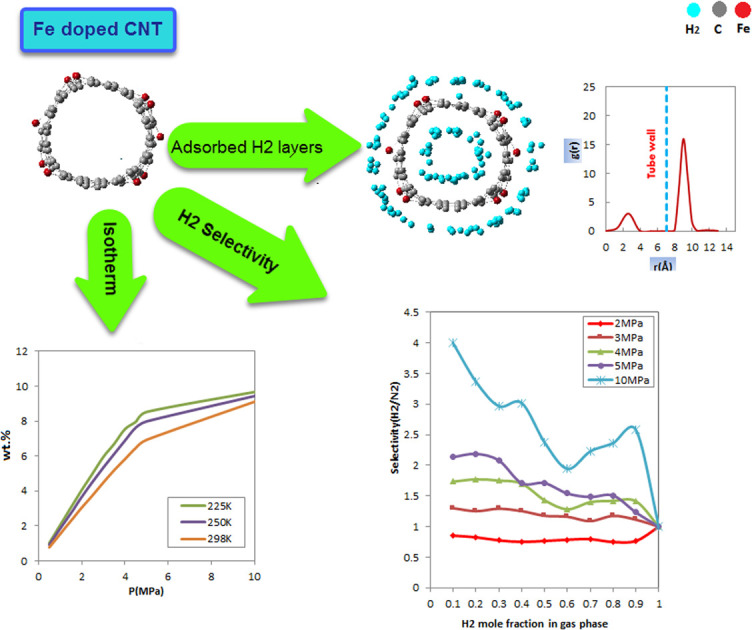
Given that adsorption is widely regarded as a favorable technique for hydrogen storage, it is appropriate to pursue the development of suitable adsorbent materials for industrial storage. This study aimed to assess the potential of Fe-doped carbon nanotubes (FeCNT) as a remarkable material for hydrogen storage. The structures of pure and Fe-doped CNTs were optimized based on quantum mechanical calculations using density functional theory (DFT) with the Perdew–Burke–Ernzerhof (PBE) method. To gain a comprehensive understanding of the adsorption behavior, Monte Carlo simulation was employed to investigate the adsorption of hydrogen molecules on FeCNT. The study specifically examined the impact of temperature, pressure, and hydrogen mole percentage on the adsorption capacity of FeCNT. The findings indicated that the uptake of hydrogen increased as the pressure increased, and when the pressure exceeded 5 MPa, FeCNT reached a state of near saturation. At room temperature and pressures of 1 and 5 MPa, the hydrogen capacities of FeCNT were determined to be 1.53 and 6.92 wt%, respectively. The radial distribution function diagrams confirmed the formation of a one-layer adsorption phase at pressures below 5 MPa. A comparison of the temperature dependence of hydrogen adsorption between FeCNT and pure CNT confirmed the effectiveness of Fe doping in hydrogen storage up to room temperature. FeCNT exhibited a greater reduction in initial hydrogen capacity at temperatures above room temperature. To evaluate the safety of the system, the use of N 2 as a dilution agent was investigated by examining the hydrogen uptake of FeCNT from pure and H 2/N 2 mixtures at 300 K. The results showed that the addition of N 2 to the environment had no significant effect on FeCNT hydrogen storage at pressures below 4 MPa. Furthermore, the study of H 2 selectivity from the H 2/N 2 mixture indicated that FeCNT demonstrated a preference for adsorbing H 2 over a wide range of bulk mole fractions at pressures of 4 and 5 MPa, suggesting that these pressures could be considered optimal. Under these conditions, Fe doping can offer an efficient and selective adsorption surface for hydrogen storage.
Abstract
To develop a suitable adsorbent material for H 2 storage, Fe doped CNT can be applied due to its improved hydrogen adsorption capacity and selective adsorption surface from the H 2/N 2 mixture.
Related collections
Most cited references118
- Record: found
- Abstract: not found
- Article: not found
Generalized Gradient Approximation Made Simple
- Record: found
- Abstract: not found
- Article: not found
Equation of State Calculations by Fast Computing Machines
- Record: found
- Abstract: not found
- Article: not found