- Record: found
- Abstract: found
- Article: not found
A Systematic Investigation of p-Nitrophenol Reduction by Bimetallic Dendrimer Encapsulated Nanoparticles

Read this article at
Abstract
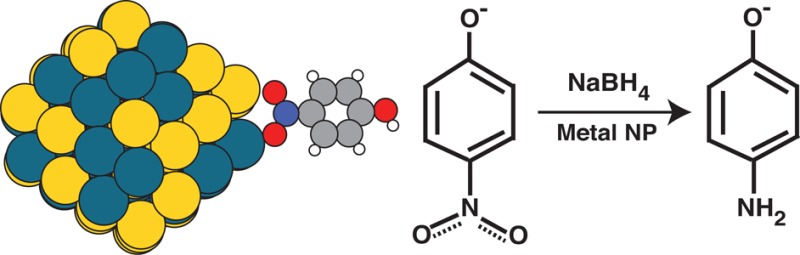
We demonstrate that the reduction of p-nitrophenol to p-aminophenol by NaBH 4 is catalyzed by both monometallic and bimetallic nanoparticles (NPs). We also demonstrate a straightforward and precise method for the synthesis of bimetallic nanoparticles using poly(amido)amine dendrimers. The resulting dendrimer encapsulated nanoparticles (DENs) are monodisperse, and the size distribution does not vary with different elemental combinations. Random alloys of Pt/Cu, Pd/Cu, Pd/Au, Pt/Au, and Au/Cu DENs were synthesized and evaluated as catalysts for p-nitrophenol reduction. These combinations are chosen in order to selectively tune the binding energy of the p-nitrophenol adsorbate to the nanoparticle surface. Following the Brønsted–Evans–Polanyi (BEP) relation, we show that the binding energy can reasonably predict the reaction rates of p-nitrophenol reduction. We demonstrate that the measured reaction rate constants of the bimetallic DENs is not always a simple average of the properties of the constituent metals. In particular, DENs containing metals with similar lattice constants produce a binding energy close to the average of the two constituents, whereas DENs containing metals with a lattice mismatch show a bimodal distribution of binding energies. Overall, in this work we present a uniform method for synthesizing pure and bimetallic DENs and demonstrate that their catalytic properties are dependent on the adsorbate’s binding energy.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Ammonia synthesis from first-principles calculations.
- Record: found
- Abstract: not found
- Article: not found
CO Chemisorption at Metal Surfaces and Overlayers
- Record: found
- Abstract: found
- Article: not found
